- Department of Neurosurgery, Hackensack Meridian School of Medicine, Nutley, United States
- Department of Neurosurgery, Hackensack University Medical Center, Hackensack, New Jersey, United States
Correspondence Address:
Elma A. Chowdhury, Department of Neurosurgery, Hackensack Meridian School of Medicine, Nutley, United States.
DOI:10.25259/SNI_707_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Elma A. Chowdhury1, Vijay Sivan1, Rohit Prem Kumar1, Francis F. Ruzicka IV1, Hooman Azmi2. Delayed clinical response to focused ultrasound thalamotomy in essential tremor in a patient with suboptimal skull density ratio – A case report. 08-Nov-2024;15:406
How to cite this URL: Elma A. Chowdhury1, Vijay Sivan1, Rohit Prem Kumar1, Francis F. Ruzicka IV1, Hooman Azmi2. Delayed clinical response to focused ultrasound thalamotomy in essential tremor in a patient with suboptimal skull density ratio – A case report. 08-Nov-2024;15:406. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13209
Abstract
Background: Magnetic resonance imaging-guided focused ultrasound (MRgFUS) thalamotomy offers incisionless treatment for essential tremor or tremor-dominant Parkinson’s disease, gaining acceptance as an alternative to deep brain stimulation. Compared to other methods, it offers real-time efficacy assessment without ionizing radiation.
Case Description: A 63-year-old male underwent MRgFUS, initially yielding subtle results due to skull limitations. However, significant tremor relief emerged 6 hours post-procedure, sustained for 5 days. Imaging confirmed thalamotomy effect. A second treatment was delivered at day five for longevity.
Conclusion: For patients with challenging skull characteristics and initial suboptimal outcomes, staged procedures may be considered, with potential delayed benefits and the need for lesion expansion for long-term relief.
Keywords: Case report, Delayed clinical response, Essential tremor, Focused ultrasound, Staging, Thalamotomy
INTRODUCTION
Magnetic resonance imaging-guided focused ultrasound (MRgFUS) thalamotomy has been Food and Drug Administration-approved for the treatment of upper extremity tremor in patients with essential tremor (ET) or tremor-dominant Parkinson’s disease (PD).[
This technique is attractive as subthreshold temperature elevations allow for reversible changes at the target and thus for “mapping” of the target area to assess for response. Temperature elevations up to 52°C allow for this reversible change. Once target assessment has been made, sonications resulting in temperatures above 55°C create permanent and irreversible changes at the target.[
A significant portion of the delivered energy is lost as the ultrasound waves traverse the skull.[
ILLUSTRATIVE CASE
Clinical presentation
A 63-year-old male with over a 20-year history of ET, with disabling tremors affecting activities of daily living and work, was referred for evaluation. Medication trials in the past were unsuccessful due to lack of efficacy, side effects, or both. He underwent testing for candidacy for either MRgFUS or deep brain stimulation (DBS) for the treatment of his tremors. A magnetic resonance imaging (MRI) of the brain was unremarkable, and a CT scan demonstrated an SDR of 0.39. Although initially offered DBS due to his borderline SDR and bilateral tremors, the patient and his family chose to proceed with MRgFUS. As a non-U.S. resident, he had concerns about long-term monitoring, maintenance, and generator changes required with DBS.
Intraoperative findings
After preparations, including placement of a stereotactic frame, he was placed on the MRI table, and after ensuring comfort and securing the head frame, a volumetric T1-weighted study was obtained. Internal landmarks were identified (AC-PC distance of 22.31), and the target coordinates were selected (X: −14.0, Y: 5.3, Z: 1.5).
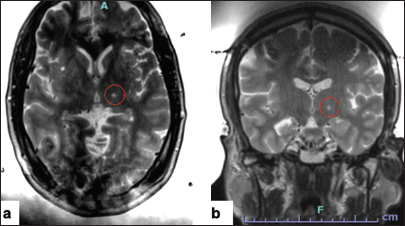
The target was moved slightly anteriorly and the parameters were set for a thalamotomy as there was some clinical improvement with the prior preceding sonication. 40,000 J were delivered, and an average temperature of 52°C was reached. The patient had mild tremor relief again with transient paresthesias. An intraoperative T2-weighted scan was obtained to assess the target [
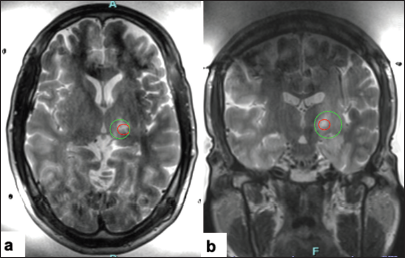
The next target was placed 1 mm anterior and 1.5 superior to the prior target to expand the lesion. The prescription was set to deliver 52,800 J to the target; however, with cavitations, only 26,000 J were delivered, and the average temperature reached only 47°C. Cavitation is a phenomenon where the propagating ultrasonic waves can expand small amounts of gas in tissues. When transient, the expanded gas bubbles collapse and release high energy, damaging surrounding tissue. The FUS technology is able to detect acoustic signatures of this phenomenon and will not proceed with further energy delivery once cavitations are detected to ensure the safety of the procedure.[
At this point, with high energy delivery being required and with a drop off in the skull efficiency, we decided to conclude the procedure. The patient had a small degree of thalamotomy effect and demonstrated some lesion formation that was visible on imaging.
Delayed findings
Six hours later, while the patient was at home, he noticed a significant improvement in the ability to use his right hand to drink and eat without any tremors. As the patient intended to leave the U.S. a week after the procedure, he was brought back on day 5 after the initial treatment for additional imaging to delineate lesion versus edema.
Second session
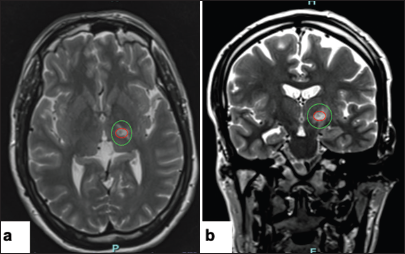
On postoperative day 5, the patient still had excellent tremor relief. Imaging revealed lesion formation and some associated edema [
DISCUSSION
Observations
In this case review, we present a patient with a delayed clinical and radiological efficacy of thalamotomy. The treatment was more challenging as the patient required significantly higher energy delivery to reach the intended temperatures, and the clinical and radiological findings during the procedure were not optimal. The decision to conclude the procedure proved fortuitous, as the patient developed a delayed arrest of the tremor 6 hours after the procedure. We confirmed radiological findings 5 days after the procedure and decided to proceed with more treatments to ensure the longevity of the results. The patient is now 9 months from his treatment with sustained benefit.
Lessons
MRgFUS is becoming more accepted as an option for addressing upper extremity tremors in those with ET and tremor-dominant PD.[
“Mapping” the target of interest with subthreshold sonications is important in assessing side effects of paresthesias related to the proximity of the sensory ventral caudal nucleus of the thalamus as well as efficacy before the creation of more permanent lesions. A successful procedure is based on the ability of the ultrasound waves to penetrate the skull and reach the intended target, with enough remaining energy to produce a rise in the temperature of the target tissue. Several skull characteristics affect this[
Another interesting observation during focused ultrasound procedures is the degradation in the efficiency of the sonications, where each subsequent sonication requires more energy delivery.[
CONCLUSION
This case report illustrates the clinical course of a patient with a delayed clinical and radiological efficacy of the treatment. This may be the first documented instance of delayed clinical improvement after MRgFUS thalamotomy, as previous literature documents typically immediate clinical benefits.[
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Hooman Azmi has a consulting agreement with Insightec as part of the Infocus program.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chang JW, Park CK, Lipsman N, Schwartz ML, Ghanouni P, Henderson JM. A prospective trial of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: Results at the 2-year follow-up. Ann Neurol. 2018. 83: 107-14
2. Chang KW, Park YS, Chang JW. Skull factors affecting outcomes of magnetic resonance-guided focused ultrasound for patients with essential tremor. Yonsei Med J. 2019. 60: 768-73
3. D’Souza M, Chen KS, Rosenberg J, Elias WJ, Eisenberg HM, Gwinn R. Impact of skull density ratio on efficacy and safety of magnetic resonance-guided focused ultrasound treatment of essential tremor. J Neurosurg. 2019. 132: 1392-7
4. Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013. 369: 640-8
5. Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016. 375: 730-9
6. Fishman PS, Elias WJ, Ghanouni P, Gwinn R, Lipsman N, Schwartz M. Neurological adverse event profile of magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor. Mov Disord. 2018. 33: 843-7
7. Gateau J, Aubry JF, Chauvet D, Boch AL, Fink M, Tanter M. In vivo bubble nucleation probability in sheep brain tissue. Phys Med Biol. 2011. 56: 7001-15
8. Halpern CH, Santini V, Lipsman N, Lozano AM, Schwartz ML, Shah BB. Three-year follow-up of prospective trial of focused ultrasound thalamotomy for essential tremor. Neurology. 2019. 93: e2284-93
9. Jung NY, Rachmilevitch I, Sibiger O, Amar T, Zadicario E, Chang JW. Factors related to successful energy transmission of focused ultrasound through a skull: A study in human cadavers and its comparison with clinical experiences. J Korean Neurosurg Soc. 2019. 62: 712-22
10. Kim C, Eames M, Paeng DG. Improving sonication efficiency in transcranial MR-guided focused ultrasound treatment: A patient-data simulation study. Bioengineering (Basel). 2023. 11: 27
11. Langford BE, Ridley CJ, Beale RC, Caseby SC, Marsh WJ, Richard L. Focused ultrasound thalamotomy and other interventions for medication-refractory essential tremor: An indirect comparison of short-term impact on health-related quality of life. Value Health. 2018. 21: 1168-75
12. Leighton TG, editors. The acoustic bubble. London: Academic Press; 1994. p.
13. Tsai KW, Chen JC, Lai HC, Chang WC, Taira T, Chang JW. The distribution of skull score and skull density ratio in tremor patients for MR-guided focused ultrasound thalamotomy. Front Neurosci. 2021. 15: 612940
14. Vetkas A, Boutet A, Sarica C, Germann J, Gwun D, Yamamoto K. Successful magnetic resonance-guided focused ultrasound treatment of tremor in patients with a skull density ratio of 0.4 or less. J Neurosurg. 2023. 140: 639-47
15. Xu Z, Carlson C, Snell J, Eames M, Hananel A, Lopes MB. Intracranial inertial cavitation threshold and thermal ablation lesion creation using MRI-guided 220-kHz focused ultrasound surgery: Preclinical investigation. J Neurosurg. 2015. 122: 152-61
16. Yamamoto K, Ito H, Fukutake S, Kamei T, Yamaguchi T, Taira T. Ventralis intermedius thalamotomy with focused ultrasound for patients with low skull density ratio. Mov Disord. 2019. 34: 1239-40
17. Yang AI, Hitti FL, Alabi OO, Joshi D, Chaibainou H, Henry L. Patient-specific effects on sonication heating efficiency during magnetic resonance-guided focused ultrasound thalamotomy. Med Phys. 2021. 48: 6588-96
18. Yuen J, Goyal A, Kaufmann TJ, Jackson LM, Miller KJ, Klassen BT. Comparison of the impact of skull density ratio with alternative skull metrics on magnetic resonance-guided focused ultrasound thalamotomy for tremor. J Neurosurg. 2023. 138: 50-7
19. Yuen J, Miller KJ, Klassen BT, Lehman VT, Lee KH, Kaufmann TJ. Hyperostosis in combination with low skull density ratio: A potential contraindication for magnetic resonance imaging-guided focused ultrasound thalamotomy. Mayo Clin Proc Innov Qual Outcomes. 2022. 6: 10-5