- Department of Neurosurgery, University of Louisville, Louisville, Kentucky, United States.
DOI:10.25259/SNI_180_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zaid Aljuboori, Mohammed Nuru, Alexandria Schaber, Haring Nauta, Emily Sieg. Delayed recurrence of acute subdural hematoma in a patient with plasminogen activator inhibitor mutation. 18-Sep-2020;11:292
How to cite this URL: Zaid Aljuboori, Mohammed Nuru, Alexandria Schaber, Haring Nauta, Emily Sieg. Delayed recurrence of acute subdural hematoma in a patient with plasminogen activator inhibitor mutation. 18-Sep-2020;11:292. Available from: https://surgicalneurologyint.com/surgicalint-articles/10270/
Abstract
Background: Plasminogen activator inhibitor type I (PAI-1) is important for balancing the fibrinolytic effect of plasmin, and deficiency can result in increased risk of bleeding. We report a case of a patient with PAI-1 deficiency who presented with delayed spontaneous recurrence of an acute subdural hematoma (aSDH) after evacuation.
Case Description: A 29-year-old male presented with altered mental status (AMS) after a fall at a construction site with Glasgow Coma Scale (GCS 4T). His coagulation profile was normal, and brain computed tomography (CT) showed a left-sided aSDH. He underwent emergent evacuation of the hematoma. On postoperative day 2, he was started on heparin for venous thromboembolism (VTE) prophylaxis. His neurological examination improved and was discharged with no focal deficits. Three days later, he presented with sudden AMS (GCS 7T); CT head showed a large hematoma at the site of original surgery. The hematoma was evacuated emergently. On readmission, the family informed providers that the patient had a history of PAI-1 deficiency. Postoperatively, only mechanical VTE prophylaxis was used and the patient was started on oral TXA per hematology recommendation. The patient improved and was discharged with no focal deficit. On follow-up, he remained neurologically stable.
Conclusion: PAI-1 deficiency should be suspected in patients with delayed posttraumatic/surgical bleeding and a normal coagulation profile. If PAI-1 deficiency is evident or suspected, then a trial of antifibrinolytic agent should be used to treat and prevent recurrence of bleeding. Furthermore, chemical VTE prophylaxis should be avoided as it increases the risk for bleeding.
Keywords: Fibrinolysis, Plasminogen activator inhibitor, Subdural, Tranexamic acid, Trauma
INTRODUCTION
Plasminogen and its activation system are an important part of the normal coagulation cascade as they promote fibrinolysis of mature thrombi.[
CASE DESCRIPTION
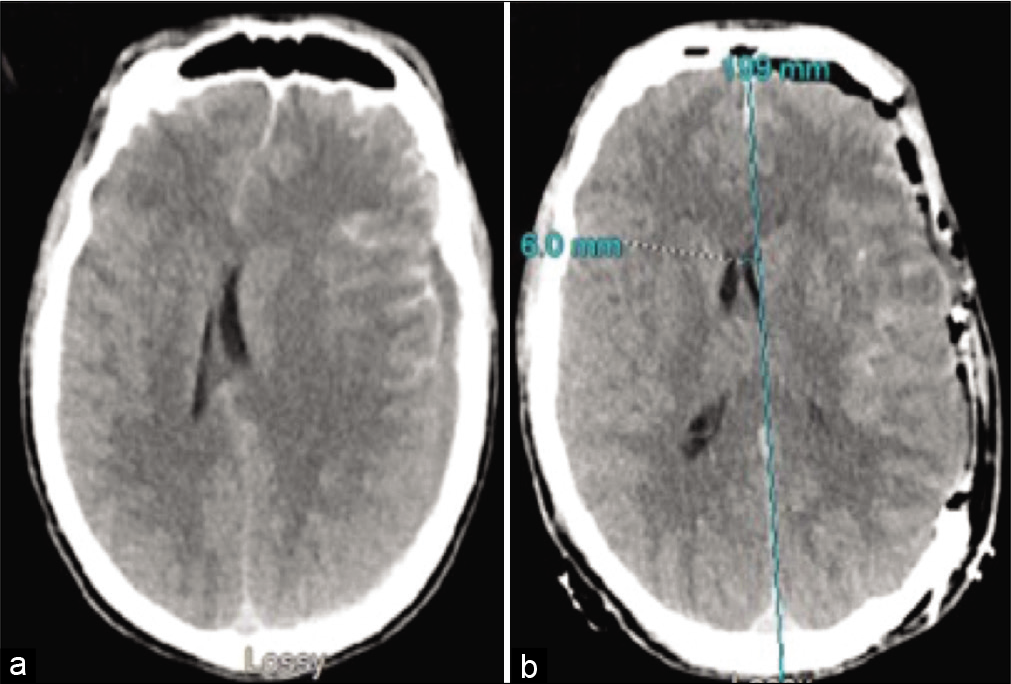
The patient is a 29-year-old Amish male who presented with altered mental status (AMS) after a fall from height. His initial Glasgow Coma Scale (GCS) was 4T with a dilated nonreactive left pupil. His computed tomography (CT) of the brain showed an acute left-sided aSDH with a 15 mm rightward shift [
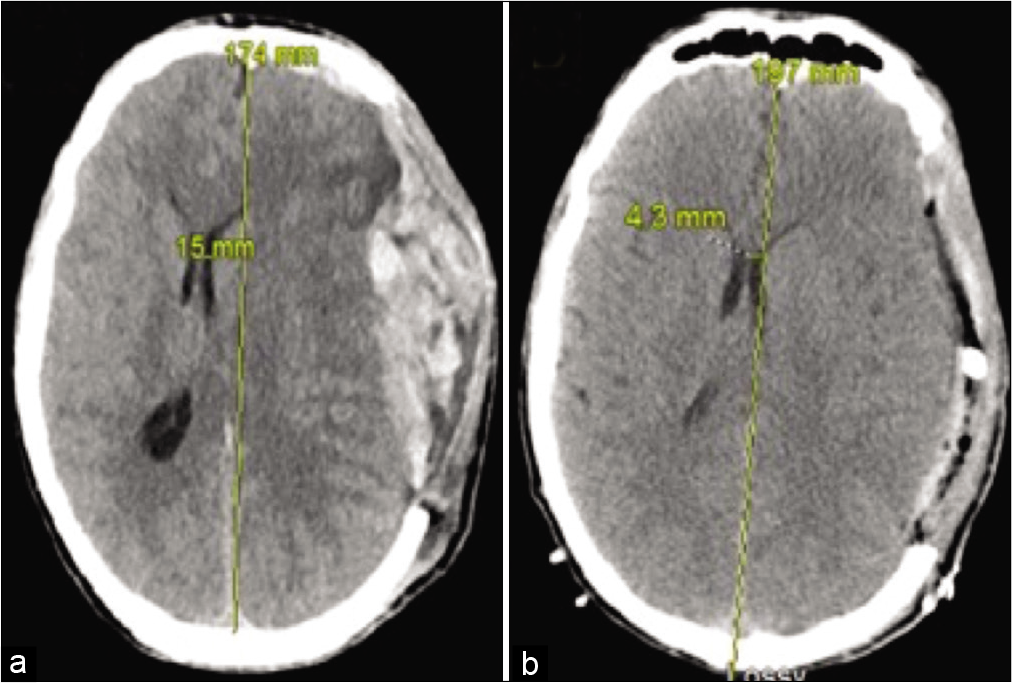
The patient presented 3 days later with sudden AMS that was preceded by a sudden headache. His GCS was 7T and CT head showed large aSDH at the site of previous surgery [
DISCUSSION
Plasmin is the primary enzyme responsible for fibrinolysis and is formed by the action of tissue plasminogen activator on plasminogen.[
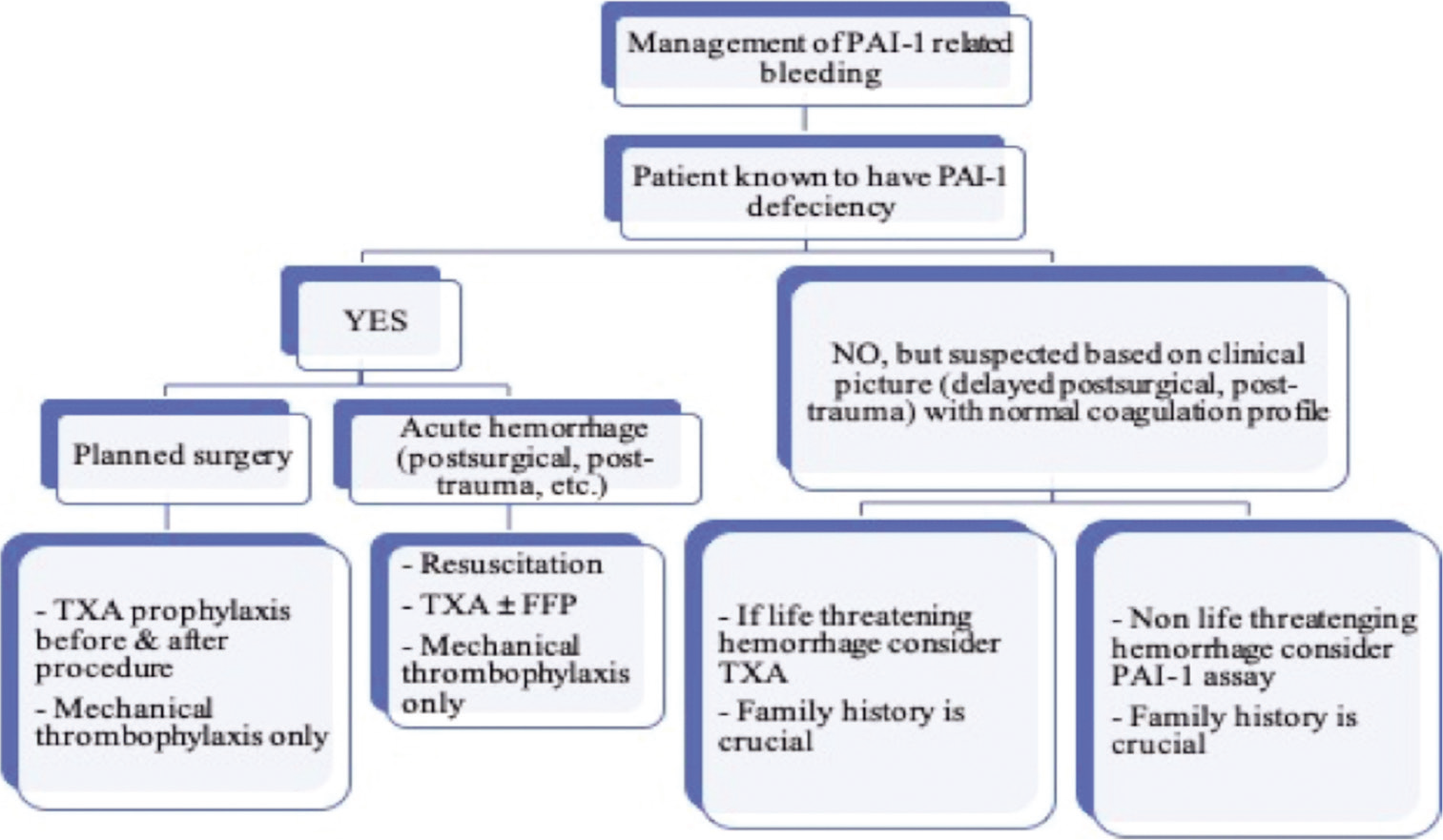
The management of patients with PAI-1 deficiency requires a multistep approach, and it is important to coordinate with the hemoncology team as they have the required expertise to guide the process [
For the treatment of acute hemorrhage (e.g., intracranial hemorrhage with or without evacuation), the administration of intravenous antifibrinolytics (e.g., TXA) is effective in controlling the bleeding in PAI-1 patients.[
The patient education regarding bleeding manifestations and when to seek treatment is important. Furthermore, patients should be offered genetic counseling, since PAI-1 deficiency is inherited in an autosomal recessive manner.[
CONCLUSION
PAI-1 deficiency is a rare hereditary bleeding disorder that was recently discovered. It should be considered in patients with delayed posttraumatic or postsurgical bleeding, especially in a population known to harbor the genetic mutation (e.g., Amish). If PAI-1 deficiency is established or highly suspected, then a trial of antifibrinolytic agents (e.g., TXA) should be considered. They can also be used as prophylaxis to prevent bleeding in patients with PAI-1 deficiency before any surgical intervention. Finally, chemical VTE prophylaxis puts the patients at risk of bleeding.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agren A, Wiman B, Schulman S. Laboratory evidence of hyperfibrinolysis in association with low plasminogen activator inhibitor Type 1 activity. Blood Coagul Fibrinolysis. 2007. 18: 657-60
2. Agren A, Wiman B, Stiller V, Lindmarker P, Sten-Linder M, Carlsson A. Evaluation of low PAI-1 activity as a risk factor for hemorrhagic diathesis. J Thromb Haemost. 2006. 4: 201-8
3. Booth NA, Bachmann F, Coleman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ.editors. Plasminogen-plasmin system. Hemostasis and Thrombosis. Basic Principles and Clinical Practice. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. p.
4. Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005. 93: 631-40
5. Heiman M, Gupta S, Khan SS, Vaughan DE, Shapiro AD, Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LH, Stephens K.editors. Complete plasminogen activator inhibitor 1 deficiency. GeneReviews. Seattle, WA: University of Washington, Seattle; 2017. p.
6. Iwaki T, Nagahashi K, Kobayashi T, Umemura K, Terao T, Kanayama N. The first report of uncontrollable subchorionic and retroplacental haemorrhage inducing preterm labour in complete PAI-1 deficiency in a human. Thromb Res. 2012. 129: e161-3
7. Kohler HP, Grant PJ. Plasminogen-activator inhibitor Type 1 and coronary artery disease. N Engl J Med. 2000. 342: 1792-801
8. Kolev K, Longstaff C. Bleeding related to disturbed fibrinolysis. Br J Haematol. 2016. 175: 12-23
9. . Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet. 2019. 394: 1713-23






Sweta Gupta, MD, MS, Pediatric hematologist
Posted December 5, 2022, 1:35 pm
We read with interest the case report titled “Delayed recurrence of acute subdural hematoma in a patient with plasminogen activator inhibitor mutation” authored by Aljuboori et al (Aljuboori Z, Nuru M, Schaber A, Nauta H, Sieg E. Delayed recurrence of acute subdural hematoma in a patient with plasminogen activator inhibitor mutation. Surg Neurol Int 2020;11:292). We would like to comment on this case report to draw attention to an error regarding the patient’s diagnosis of Plasminogen activator inhibitor deficiency (PAI-1). This patient is well known to our institution, the Indiana Hemophilia and Thrombosis Center (IHTC), in Indianapolis, Indiana. We diagnosed the proband within this community with PAI-1 deficiency and subsequently have done extensive evaluation of individuals within this pedigree. The mutation in this family has been described as a rare frame shift mutation (c.699_700dupTA) in the SERPINE1 (1); the homozygous state results in complete deficiency of PAI-1 which is clinically manifested as moderate to severe bleeding phenotype (1,2).
This patient has previously reported prolonged gingival oozing for 6 weeks post dental extraction and excessive bleeding with cuts and wounds. As previously stated, he has a well-documented family history of both heterozygous (unaffected) and homozygous (affected) PAI-1 deficiency. The heterozygous or carrier state of this mutation does not result in bleeding symptoms even with invasive procedures. Our center has followed >100 PAI-1 heterozygotes for a period of approximately three decades with no reported bleeding symptoms in any individuals within this cohort. This distinction is an important one to make as the patient described in the case report is heterozygous for the (c.699_700dupTA) mutation. He was tested by us in 2015 and reconfirmed in 2020 during the management of the subdural hematoma. Within this patient’s family, there are siblings and cousins with complete PAI-1 deficiency with a well-documented moderate bleeding phenotype including post-surgical, post-traumatic bleeding, development of hematomas with injury, heavy menstrual bleeding, antepartum and post-partum bleeding; all known affected individuals within this family are followed closely by our center and treated as needed (1, 2, 3).
The plan for post operative surgical management in this patient utilizing an antifibrinolytic agent, tranexamic acid, was recommended by us based on the bleeding phenotype as antifibrinolytic agents are universal hemostatic agents (4, 5); as yet a definitive diagnosis as a cause for this patient’s bleeding has not been established. Further hemostatic evaluation has been planned for this patient.
Additionally, we would like to emphasize the drawbacks of routine PAI-1 laboratory testing (antigen and activity) which include lack of standardization across laboratories, level fluctuation based on diurnal variation, and difficulty utilizing the activity assay lower limit as a diagnosis as normal individuals may have PAI-1 activity levels below the laboratory’s detection level ( 6, 7). These issues increase the diagnostic difficulty of PAI-1 deficiency. A normal euglobulin clot lysis time (ECLT) used as a screening test for the fibrinolytic pathway, rules out dysfunctional PAI-1 and alpha-2 antiplasmin deficiency, with a reasonable degree of certainty (8,9).
Although it’s intuitive to conclude that the rare family bleeding disorder of complete PAI-1 deficiency might be the explanation for bleeding in our patient, we would want to emphasize that his clinical presentation cannot be attributed to PAI-1 deficiency as this patient is heterozygous for the mutation.
References:
Fay WP, Shapiro AD, Shih JL, Schleef RR, Ginsburg D. Brief report: complete deficiency of plasminogen-activator inhibitor type 1 due to a frame-shift mutation. N Engl J Med 1992;327:1729-33.
Fay W, Parker A, Condrey L, Shapiro AD. Human plasminogen activator inhibitor-1 (PAI-1) deficiency: characterization of a large kindred with a null mutation in the PAI-1 gene. Blood 1997;90:204-8.
Heiman M, Gupta S, Shapiro AD. The obstetric, gynaecological and fertility implications of homozygous PAI-1 deficiency: single-centre experience. Haemophilia 2014;20:407-12.
Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: An illustrated review. Res Pract Thromb Haemost. 2021;14;5(5):e12546.
Tengborn L, Blombäck M, Berntorp E. Tranexamic acid–an old drug still going strong and making a revival. Thromb Res. 2015;135(2):231-42
Mehta R, Shapiro AD. Plasminogen activator inhibitor type 1 deficiency. Haemophilia 2008;14:1255-60.
Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation 1989;79:101-6.
Ilich A, Bokarev I, Key NS. Global assays of fibrinolysis. Int J Lab Hematol. 2017;39(5):441-447
Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemost 2018; 16: 652–662.