- Department of Neurosurgery, Keio University School of Medicine, Shinjuku, Tokyo, Japan.
DOI:10.25259/SNI_235_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Satoshi Takahashi, Kazunari Yoshida. Delayed reopening of a superficial temporal artery to middle cerebral artery bypass graft occluded by a white thrombus during surgery. 01-Aug-2020;11:220
How to cite this URL: Satoshi Takahashi, Kazunari Yoshida. Delayed reopening of a superficial temporal artery to middle cerebral artery bypass graft occluded by a white thrombus during surgery. 01-Aug-2020;11:220. Available from: https://surgicalneurologyint.com/surgicalint-articles/10174/
Abstract
Background: To the authors’ knowledge, reopening of a superficial temporal artery to middle cerebral artery (STA-MCA) bypass graft occluded by a white thrombus during the procedure and was observed several months after the surgery is relatively rare.
Case Description: The authors encountered a case of moyamoya disease in an Asian female in her third decade of life, in whom a bypass recipient vessel was occluded by a white thrombus during surgery and remained occluded on magnetic resonance angiography (MRA) performed up to 6 weeks after the procedure. However, recanalization was confirmed by MRA performed 4 months after surgery. MRA performed 10 and 19 months after surgery revealed that the bypass vessel had grown thicker, and the ischemic symptoms experienced by the patient also improved.
Conclusion: Whether this lesion is explained by reopening or angiogenesis, its pathophysiology remains controversial. The uninterrupted connection of occluded bypass vessel in STA-MCA bypass surgery in conjunction with surgical strategy of single bypass using only parietal branch of STA as donor and preserving blood flow of frontal branch to scalp may have made a positive impact on promoting the development of extracranial-intracranial bypass anastomosis in the chronic phase and should be considered.
Keywords: Bypass surgery, Complication, Moyamoya disease, Reopening, White thrombus
INTRODUCTION
Moyamoya disease is a relatively rare cerebrovascular clinical entity characterized by progressive occlusion of the terminal portion of the supraclinoid internal carotid artery (ICA) and its main branches within the circle of Willis. This occlusion results in the formation of a fine vascular network known as “moyamoya vessels” at the basal ganglia.[
Recently, we treated a patient with moyamoya disease in whom reopening of an superficial temporal artery to middle cerebral artery (STA-MCA) bypass graft occluded by a white thrombus during surgery was observed several months after the procedure. In the present report, we discuss the pathophysiology of the case we encountered and compare it with previous similar reports to further understand the phenomenon.
CASE REPORT
History and examination
An Asian woman in her third decade of life complained of numbness in the right side of her body and difficulties with manual dexterity since childhood; however, intracranial radiological screening was not performed. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) of the head were performed for the 1st time due to attacks of weakness and vertigo, in addition to her initial symptoms. MRA revealed severe stenosis at the terminal portion of the bilateral ICAs, and she was diagnosed with suspected moyamoya disease and was referred to the authors’ hospital for further evaluation and treatment. On admission, she was alert and exhibited no apparent neurological sequelae such as motor weakness. Cerebral angiography revealed marked stenosis at the terminal portion of the ICA. No anterior and middle cerebral arteries were visible, the left posterior cerebral artery had begun to fall off, and moyamoya vessels were present in the arterial phase in the region of basal ganglia. The patient was diagnosed with moyamoya disease, Suzuki Grade 4. Although spontaneous EC-IC anastomosis through the bilateral middle meningeal arteries (MMAs) was confirmed, extensive lowering of blood flow in the left cerebral hemisphere was confirmed using single-photon emission computed tomography. Her symptoms were compatible with those of ischemia of moyamoya disease. Accordingly, direct plus indirect bypass surgery was planned.
Surgery
The patient was taking cilostazol during the perioperative period. Because ischemic attacks had been repeated before surgery, cilostazol was continued until the day of surgery. In the perioperative period, antiplatelet drugs are always used as a single agent (usually low-dose aspirin) in our usual practice. Normocapnia and normotension were requested to the anesthesiologist in charge of the operation. A “U” shape skin incision surrounding the parietal branch of the STA, which was marked in the echo guide before surgery, was performed, and the parietal branch of the STA was harvested from the backside of the skin flap. A bone flap, 8 cm×6 cm in size, was then removed, taking care not to injure the MMA. A dural incision was performed, leaving the MMA-like leaf vein so as not to injure the spontaneous EC-IC anastomosis through MMA. When indocyanine green angiography was performed to confirm M4 arteries on the brain surface, only a single M4 artery was considered to be a candidate for a recipient artery (0.7 mm in diameter). After trimming the ends of the donor artery (STA) using a “fish-mouth” technique, the anastomosis site of M4 was occluded using temporary clips. An arteriotomy was placed and the donor STA was sutured to the recipient M4 using 11 10-0 nylon stitches. After removing the temporary clips, the site of bypass anastomosis was morphologically expanded [
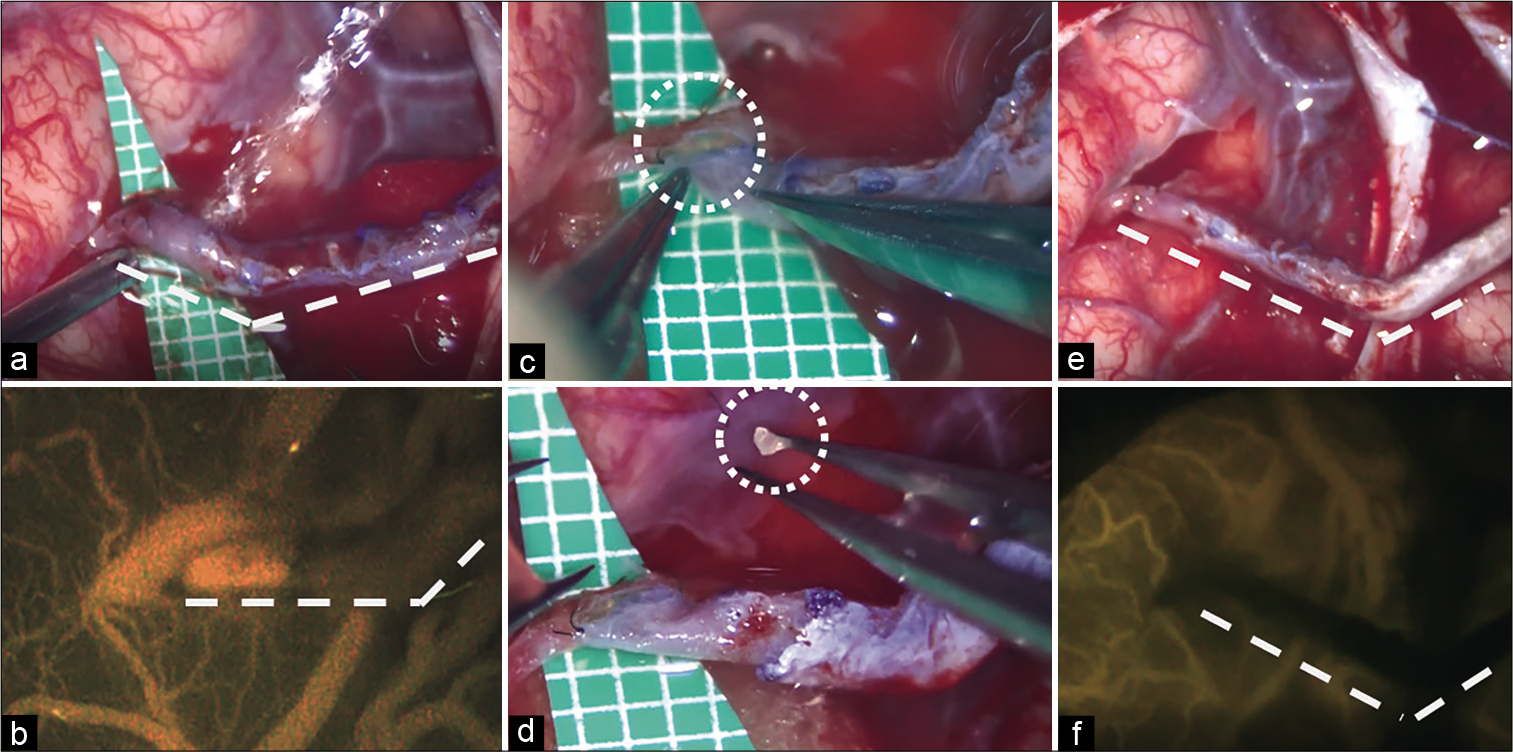
Figure 1:
Intraoperative photographs. The site of bypass anastomosis was initially morphologically expanded (a), and the good blood flow from the bypass was confirmed (b). All five stitches on one side of anastomosis were removed to confirm the lumen of anastomosis site (c). In the lumen, a white thrombus was revealed, as expected (d). The superficial temporal artery was anastomosed to the occluded recipient at the end of the surgery (e and f).
Trouble shooting
Along with attenuation of blood flow through the bypass, the color tone of the recipient M4 artery changed to a whitish color, suggesting the formation of a white thrombus. With these findings, the authors gently grasped the anastomosis site using forceps expecting to mechanically break down the thrombus. With this maneuver, it was observed that the color tone temporarily improved to reddish, and temporal recanalization was confirmed by Doppler echo; nevertheless, complete recanalization was not achieved. Therefore, 3000 units of heparin were intravenously injected in conjunction with administration of 100 mg of aspirin through nasogastric tube which were performed to affect thrombolysis. Despite these measures, recanalization of the bypass was not achieved. All five stitches on one side of anastomosis were removed to confirm the lumen of anastomosis site. In the lumen, a white thrombus was revealed, as expected [
Postoperative course
After surgery, the patient emerged from anesthesia smoothly, and no apparent neurological deficit was recognized. However, 50 h after surgery, the patient complained of difficulty with speaking, and motor aphasia was detected on neurological examination. However, although she exhibited a tiny round high intensity area on diffusion-weighted MRI, suggestive of white thrombus itself at the anastomotic site, no apparent acute cerebral infarction nor hemorrhage responsible for the symptom was detected on MRI. Her motor aphasia was diagnosed as a symptom of ischemic symptoms at the craniotomy site (which may have, in part, been due to the effect of heparin used during the operation, in which a thin subdural hematoma developed and caused slight compression to the brain surface). She was treated conservatively, the symptom of motor aphasia virtually disappeared by postoperative week 2, and she was discharged from hospital without apparent neurological sequelae.
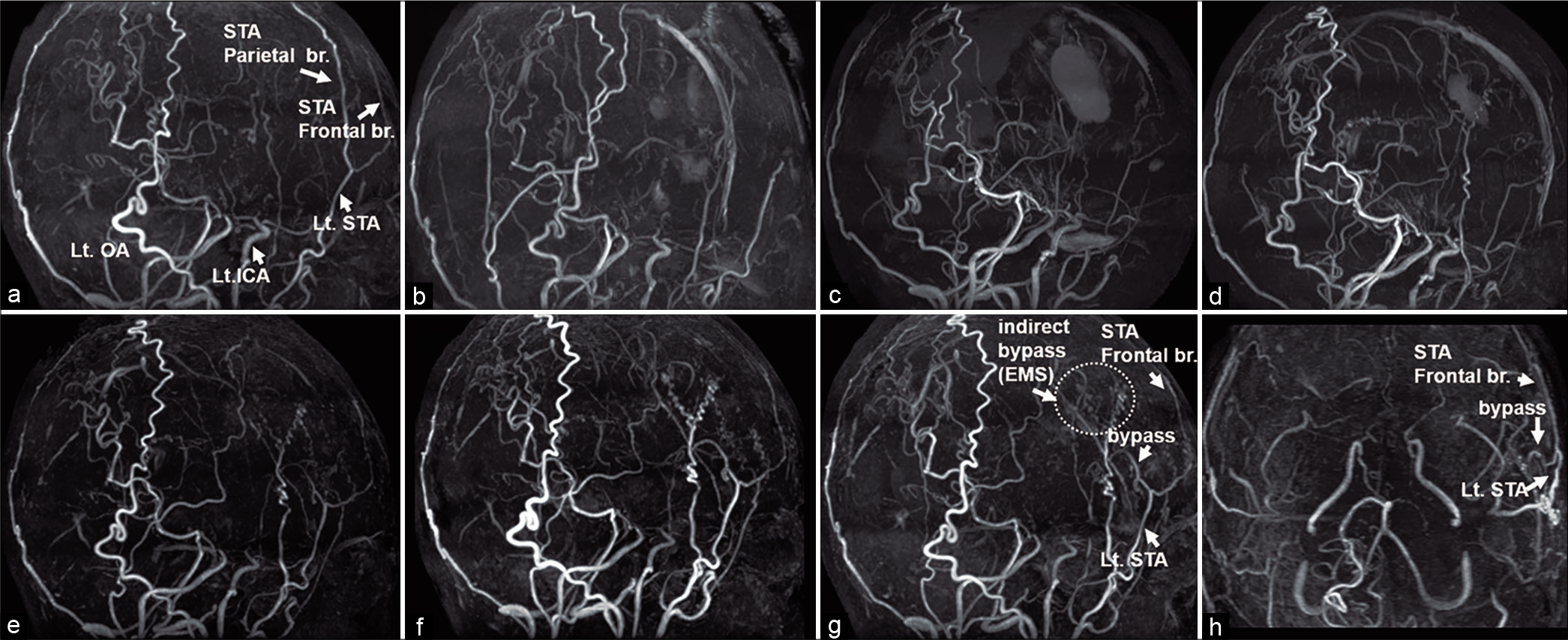
MRI + MRA were performed on an outpatient basis after discharge. The intraoperative occluded bypass vessels (using parietal branch of STA [
Figure 2:
Serial follow-up magnetic resonance angiography (MRA). Preoperative MRA (a). Intraoperative occluded bypass vessel was not visualized on magnetic resonance imaging (MRI) at 2 days (b), 2 weeks (c), and 6 weeks (d). However, the bypass vessel was detected on MRI performed 4 months after surgery (e). Furthermore, the bypass vessels became more clearly visible on MRI performed 10 months (f) and 19 months (g and h (from caudal)) after surgery (d). (Lt.: left, ICA: Internal carotid artery, STA: Superficial temporal artery, br.: Branch, OA: Occipital artery, EMS (encephalo-myo-synangiosis).
DISCUSSION
To the best of our knowledge, reopening of STA-MCA bypass graft occluded by a white thrombus during surgery was observed several months after the procedure is relatively rare.
Mikami et al. reported predictive factors for acute thrombogenesis occurring immediately after bypass procedure for moyamoya disease and found that MRA scores by Houkin et al.[
A case series reported by Kim et al. described patients with an occluded anastomosis not intraoperatively but occluded anastomosis confirmed 1 day postoperatively. In their series, there were 11 nonpatent anastomoses in 31 cases they operated.[
In contrast, Scharf et al. reported they found reopening of only 7 of 23 (30.4%) patients with occluded STA-MCA bypass at early stage after surgery.[
In their series, Kim et al. reported a patient in which intraoperative bypass obstruction was confirmed, similar to our case.[
In the present case, the delayed recanalization of EC-IC bypass was confirmed 4 months after the operation in the same region as the initially occluded bypass. In this regard, the question arises whether the occluded and recanalized vessels are, in fact, the same vessels. One possible explanation is that an occluded bypass vessel, as well as the surrounding connective tissue, became a scaffold for the newly developed artery. Even if that was true, we speculate that our strategy of uninterrupted connection of occluded bypass vessel in the surgery in conjunction with surgical strategy of single bypass using only parietal branch of STA as donor and preserving blood flow of frontal branch to scalp may have made a positive impact on promoting bypass vessel using occluded bypass vessel, as well as the surrounding connective tissue as a scaffold for the newly developed artery. In addition to this possibility, a small amount of blood flow may have remained that could not be depicted on postoperative MRA, which may have led to sufficient blood flow to be depicted on MRA with the development of bypass vessels. Because the frontal branch of the STA is preserved, and only the parietal branch is used as a bypass donor, blood flow to the bifurcation of the frontal branch and parietal branch of the STA was, at least, maintained. As such, it is considered that the pressure gradient on the occlusive blood vessel may have also led to the reopening of the occluded bypass. In summary, for both explanations for delayed recanalization, we think surgical strategy of single bypass using only parietal branch of STA as donor and preserving blood flow of frontal branch to scalp may have made a positive impact on promoting the development of EC-IC bypass anastomosis in the chronic phase.
Nevertheless, leaving an occluded resutured anastomosis raises the risk for postoperative hemorrhage from the anastomosis site, especially when the thrombus dissolves and the bypass artery recanalizes in the postoperative early stage, postoperative hemorrhage may occur from the anastomosis site. Careful suturing of the anastomosis site is required more than when patency of the bypass is confirmed.
CONCLUSION
Although the underlining pathophysiology remains controversial, uninterrupted connection of bypass vessels that occlude during surgery may promote the development of EC- IC anastomosis in the chronic phase and should be considered.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Fujimura M, Bang OY, Kim JS. Moyamoya disease. Front Neurol Neurosci. 2016. 40: 204-20
2. Houkin K, Nakayama N, Kuroda S, Nonaka T, Shonai T, Yoshimoto T. Novel magnetic resonance angiography stage grading for moyamoya disease. Cerebrovasc Dis. 2005. 20: 347-54
3. Kim SH, Lee H, Yoo M, Jin S, Lee S, Choi BS. Angiographic and clinical outcomes of non-patent anastomosis after bypass surgery in adult moyamoya disease. Acta Neurochir (Wien). 2019. 161: 379-84
4. Kuroda S, Houkin K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008. 7: 1056-66
5. Mikami T, Suzuki H, Ukai R, Komatsu K, Akiyama Y, Wanibuchi M. Predictive factors for acute thrombogenesis occurring immediately after bypass procedure for moyamoya disease. Neurosurg Rev. 2020. 43: 609-17
6. Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: Results of the Japan adult moyamoya trial. Stroke. 2014. 45: 1415-21
7. Scharf J, Schmiedek P, Kemmling A, Gerigk L, Groden C, Horn P. Spontaneous recanalization of occluded standard extracranial-intracranial arterial bypass. Cerebrovasc Dis. 2007. 23: 175-80
8. Takahashi S, Tanizaki Y, Kimura H, Akaji K, Nakazawa M, Yoshida K. Hemodynamic stress distribution reflects ischemic clinical symptoms of patients with moyamoya disease. Clin Neurol Neurosurg. 2015. 138: 104-10