- Department of Neurosurgery, University of Illinois at Chicago, Chicago, Illinois 60612, USA
Correspondence Address:
Oriela Rustemi
Department of Neurosurgery, University of Illinois at Chicago, Chicago, Illinois 60612, USA
DOI:10.4103/2152-7806.170029
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rustemi O, Alaraj A, Shakur SF, Orning JL, Du X, Aletich VA, Amin-Hanjani S, Charbel FT. Detection of unruptured intracranial aneurysms on noninvasive imaging. Is there still a role for digital subtraction angiography?. Surg Neurol Int 20-Nov-2015;6:175
How to cite this URL: Rustemi O, Alaraj A, Shakur SF, Orning JL, Du X, Aletich VA, Amin-Hanjani S, Charbel FT. Detection of unruptured intracranial aneurysms on noninvasive imaging. Is there still a role for digital subtraction angiography?. Surg Neurol Int 20-Nov-2015;6:175. Available from: http://surgicalneurologyint.com/surgicalint_articles/detection-of-unruptured-intracranial-aneurysms-on-noninvasive/
Abstract
Background:To determine the utility of digital subtraction angiography (DSA) in patients with unruptured intracranial aneurysms (UIA) detected on noninvasive imaging, such as magnetic resonance angiography (MRA) and computed tomography angiography (CTA). The follow-up of patients with untreated UIAs involves serial imaging; however, this diagnosis may be based on false positive (FP) results. We examined the incidence of FPs in our institutional series.
Methods:DSAs performed at our institution from January 2011 to June 2014 were retrospectively reviewed and patients referred with UIA detected on noninvasive imaging were selected. Clinical presentation as well as aneurysm location, size, and number reported on DSA and noninvasive imaging were assessed.
Results:Two hundred and eighty six patients (mean age 56.8 years, female 74.8%) with a total of 355 UIA were included. Thirty-one patients had a symptomatic presentation. Analysis per patient showed the pooled FP rate of noninvasive imaging was 15%. MRA FP was 13% (22/171) and CTA FP was 18% (22/120). FP increased significantly with aneurysm size P P = 0.01). Mean aneurysm size among symptomatic patients was significantly larger (P
Conclusion:DSA detection of FP UIA diagnosed on noninvasive imaging is significantly higher for aneurysms
Keywords: Angiography, cerebral aneurysm, computed tomography, digital subtraction angiography, magnetic resonance imaging
INTRODUCTION
Noninvasive imaging modalities, such as magnetic resonance angiography (MRA) and computed tomography angiography (CTA), are highly sensitive in detecting unruptured intracranial aneurysms (UIA).[
MATERIALS AND METHODS
Patient selection
Following institutional review board approval, all DSA performed at our institution from January 2011 to June 2014 were retrospectively reviewed. During this period, a total of 1395 patients had at least one DSA for various indications. Patients with at least one UIA on noninvasive CTA/MRA (predominantly performed at other institutions) were included. In patients who had both CTA and MRA performed, only the study showing a positive finding was included in the analysis.
Data collection
The location, size, and number of UIA detected by DSA and CTA/MRA were obtained from the imaging reports if available. Clinical data, including presentation and family history of cerebral aneurysm or subarachnoid hemorrhage, was determined from patient charts.
The data were initially analyzed per patient, meaning the aneurysm for which the patient was referred based on noninvasive imaging. The data were then analyzed per aneurysm. In this analysis per aneurysm, secondary false negative (FN) data (aneurysms detected on DSA but not detected on noninvasive imaging) were also obtained.
Statistical analysis
The data were analyzed using SAS 9.4 software (SAS version 9.4; SAS Institute, Cary, NC). Continuous variables were expressed as a mean ± standard deviation. A paired t-test was used for the analysis of aneurysm size associated with FP findings on CTA/MRA. Fisher's exact test was used to investigate the location of FPs on noninvasive imaging. The inaccuracy of aneurysm detection on noninvasive imaging (“secondary” FNs and FPs) versus DSA was also analyzed using Fisher's exact test. Chi-square was used to determine the accuracy of detection of multiple aneurysms on noninvasive imaging versus DSA. A P ≤ 0.05 was considered as statistically significant.
RESULTS
Statistical analysis per patient
Angiograms performed on 1395 patients over a period of 3.5 years were retrospectively reviewed. A total of 286 patients with CTA (n = 120) or MRA (n = 171) imaging were included; 7 patients had both studies performed, but with 2 patients having a negative CTA and positive MRA - only the imaging showing a positive finding was included in the analysis. Aneurysm size on CTA was available in 85 patients and on MRA in 110 patients. These patients harbored a total of 317 aneurysms visualized on CT, MRA, or both. Average age was 56.8 years ± 13.4 (range 3–89 years). About 74.8% were female (214/286). Average age did not differ between females (56.8 years ± 13.1, range 3–80 years) and males (56.9 years ± 14.3, range 12–89 years).
When compared with the aneurysms detected on CTA/MRA, DSA showed negative results in 43 patients leading to a pooled FP rate of 15% on noninvasive imaging. FP rate on MRA was 13% (22/171) and on CTA was 18% (22/120) [Tables
There were 31 symptomatic patients with a mean aneurysm size on DSA of 17.8 ± 11.3 mm (range 3–50 mm). Incidental aneurysms had a significantly smaller mean size of 7.7 ± 4.3 mm (P < 0.001). Symptoms included ophthalmoplegia (16 patients), cranial nerve III palsy (7 patients), other compression symptoms (5 patients), and embolic transient ischemic attacks (TIAs) (3 patients).
About 95% (19/20) of patients with a family history of intracranial aneurysm or subarachnoid hemorrhage (SAH) were found to have an aneurysm on DSA, which was not significantly different than the 84% (224/266) of patients without a family history (P = 0.19). Mean aneurysm size on DSA in patients with a family history of aneurysmal SAH was 6.2 ± 6.7 mm, as compared to 9.2 ± 3.8 mm in patients with a negative family history.
No location was significantly susceptible to false detection of aneurysms on noninvasive imaging. Anterior versus posterior circulation location was not significantly associated with FPs (P = 0.133). In addition, DSA detected more multiple aneurysms than noninvasive imaging (P = 0.0001).
Statistical analysis per aneurysm
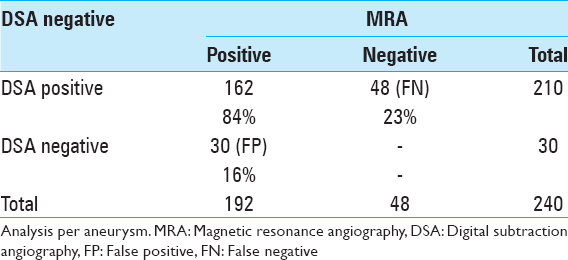
The FP rate of aneurysms detected by MRA was 16% (30/192) and the “secondary” FN rate (aneurysms detected on DSA and not detected on MRA) was 23% (48/210) [
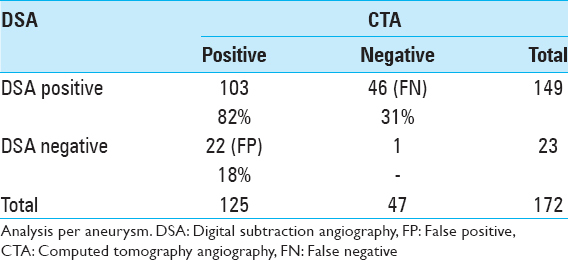
The mean size of aneurysms detected on MRA and confirmed on DSA was 9.0 ± 7.0 mm. MRA FP increased significantly for aneurysms ≤ 3.5 mm (P < 0.0001). The mean size of aneurysms detected on CTA and confirmed on DSA was 7.9 ± 5.2 mm. CTA FP increased significantly for aneurysms ≤4.0 mm (P = 0.01).
DISCUSSION
The sensitivity and specificity of noninvasive CTA/MRA imaging in the detection of UIA have been previously described in the literature.[
In our study, we found that MRA and CTA FP were 13% and 18%, respectively. We also found that MRA FP increased significantly for aneurysms smaller than 3.5 mm (P < 0.001) and that CTA FP increased significantly for aneurysms smaller than 4.0 mm (P = 0.01). These results are consistent with previous reports of decreased accuracy in the detection of UIA <5 mm by both MRA and CTA.[
Our data suggest that DSA does have a diagnostic role for small aneurysms. While DSA is considered as the gold standard for detecting and imaging UIA, it remains an invasive procedure. The risk of DSA is generally reported to be 1–2%.[
Analysis of our data per aneurysm yielded similar results to our analysis per patient, with FP of 16% on MRA and 18% on CTA and a “secondary” FN of 23% and 31%, respectively. Given our study design, FN could not be determined directly from the data, but we defined “secondary” FN as UIA detected on DSA that were not detected on noninvasive imaging.
Not surprisingly, we found that the accuracy of noninvasive imaging modalities increases for larger aneurysms, especially when symptomatic. Aneurysm size was significantly larger among symptomatic versus asymptomatic patients in our cohort (17.8 mm vs. 7.7 mm, P < 0.001). Although symptomatic aneurysms were significantly larger, small aneurysms in critical locations can be symptomatic, such as the 3 mm posterior communicating artery aneurysm in one of our patients who presented with a cranial nerve III palsy. The role of DSA for large aneurysms remains primarily adjunctive rather than diagnostic, providing more detailed morphologic characteristics to guide surgical or endovascular treatment approaches.
Limitations
The primary limitation of this study is the potential for selection bias, given that not all patients with aneurysms detected on noninvasive imaging at our institution underwent confirmatory DSA. As such, it is possible that those selected to undergo DSA may have more frequently been cases where the presence of an aneurysm was already under question, thus, leading to a higher apparent rate of FP findings. Thus, the true rate of FP may be over estimated in our analysis. Another shortcoming of this study is its retrospective design; measures of sensitivity and specificity could not be obtained directly from the data given that we only included patients with positive CTA or MRA findings. However, our primary focus was to determine the rate with which positive noninvasive imaging may be misleading rather than to determine sensitivity rates. We did attempt to address this aspect by examining “secondary” FN in patients with multiple aneurysms. Most noninvasive scans in our study were performed at outside facilities employing variable techniques, thereby possibly compromising the homogeneity and comparability of our data. At the same time, however, our results address the real world concern of patients referred from other facilities with small UIA detected on noninvasive imaging and provide insight to help guide the management of these patients.
CONCLUSION
DSA detection of FP UIA diagnosed on noninvasive imaging is significantly higher for aneurysms <4.0 mm. Accurate diagnosis with DSA may eliminate the need for further follow-up and its associated negative psychological and economic effects. Within the limitations of this retrospective study, we conclude that DSA has a diagnostic role in small aneurysms detected on noninvasive imaging.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Backes D, Vergouwen MD, Tiel Groenestege AT, Bor AS, Velthuis BK, Greving JP. PHASES score for prediction of intracranial aneurysm growth. Stroke. 2015. 46: 1221-6
2. Backes D, Vergouwen MD, Velthuis BK, van der Schaaf IC, Bor AS, Algra A. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke. 2014. 45: 1299-303
3. Chen W, Yang Y, Xing W, Qiu J, Peng Y. Application of multislice computed tomographic angiography in diagnosis and treatment of intracranial aneurysms. Clin Neurol Neurosurg. 2010. 112: 563-71
4. Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: A meta-analysis. Stroke. 1999. 30: 317-20
5. Hoh BL, Cheung AC, Rabinov JD, Pryor JC, Carter BS, Ogilvy CS. Results of a prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery. 2004. 54: 1329-40
6. Kashiwazaki D, Kuroda S. Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke. 2013. 44: 2169-73
7. Leffers AM, Wagner A. Neurologic complications of cerebral angiography. A retrospective study of complication rate and patient risk factors. Acta Radiol. 2000. 41: 204-10
8. Li MH, Li YD, Gu BX, Cheng YS, Wang W, Tan HQ. Accurate diagnosis of small cerebral aneurysms ≤5 mm in diameter with 3.0-T MR angiography. Radiology. 2014. 271: 553-60
9. Maslehaty H, Ngando H, Meila D, Brassel F, Scholz M, Petridis AK. Estimated low risk of rupture of small-sized unruptured intracranial aneurysms (UIAs) in relation to intracranial aneurysms in patients with subarachnoid haemorrhage. Acta Neurochir (Wien). 2013. 155: 1095-100
10. Menke J, Larsen J, Kallenberg K. Diagnosing cerebral aneurysms by computed tomographic angiography: Meta-analysis. Ann Neurol. 2011. 69: 646-54
11. Pradilla G, Wicks RT, Hadelsberg U, Gailloud P, Coon AL, Huang J. Accuracy of computed tomography angiography in the diagnosis of intracranial aneurysms. World Neurosurg. 2013. 80: 845-52
12. Sailer AM, Wagemans BA, Nelemans PJ, de Graaf R, van Zwam WH. Diagnosing intracranial aneurysms with MR angiography: Systematic review and meta-analysis. Stroke. 2014. 45: 119-26
13. Schwab KE, Gailloud P, Wyse G, Tamargo RJ. Limitations of magnetic resonance imaging and magnetic resonance angiography in the diagnosis of intracranial aneurysms. Neurosurgery. 2008. 63: 29-34
14. Tomycz L, Bansal NK, Hawley CR, Goddard TL, Ayad MJ, Mericle RA. “Real-world” comparison of non-invasive imaging to conventional catheter angiography in the diagnosis of cerebral aneurysms. Surg Neurol Int. 2011. 2: 134-
15. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
16. . Unruptured intracranial aneurysms - Risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998. 339: 1725-33
17. Wang H, Li W, He H, Luo L, Chen C, Guo Y. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: Comparison with conventional digital subtraction angiography. Clin Radiol. 2013. 68: e15-20
18. White PM, Teasdale EM, Wardlaw JM, Easton V. Intracranial aneurysms: CT angiography and MR angiography for detection prospective blinded comparison in a large patient cohort. Radiology. 2001. 219: 739-49
19. 19 White PM, Wardlaw JM, Easton V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology. 2000. 217: 361-70
20. Wiebers DO, Whisnant JP, Huston J 3 r rd, Meissner I, Brown RD, Piepgras DG. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10
21. Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: Prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003. 227: 522-8