- School of Medicine, University of California, San Francisco, California, USA
- Department of Neurological Surgery, University of California, San Francisco, CA, USA
- Department of Neurological Surgery, Northwestern University, Chicago, Illinois, USA
Correspondence Address:
Michel Kliot
Department of Neurological Surgery, Northwestern University, Chicago, Illinois, USA
DOI:10.4103/2152-7806.189299
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Birk H, Zygourakis CC, Kliot M. Developing an algorithm for cost-effective, clinically judicious management of peripheral nerve tumors. Surg Neurol Int 26-Aug-2016;7:80
How to cite this URL: Birk H, Zygourakis CC, Kliot M. Developing an algorithm for cost-effective, clinically judicious management of peripheral nerve tumors. Surg Neurol Int 26-Aug-2016;7:80. Available from: http://surgicalneurologyint.com/surgicalint_articles/developing-algorithm-cost%e2%80%91effective-clinically-judicious-management-peripheral-nerve-tumors/
Abstract
Peripheral nerve tumors such as neurofibromas and schwannomas have become increasingly identified secondary to improved imaging modalities including magnetic resonance neurogram and ultrasound. Given that a majority of these peripheral nerve tumors are benign lesions, it becomes important to determine appropriate management of such asymptomatic masses. We propose a normal cost-effective management paradigm for asymptomatic peripheral nerve neurofibromas and schwannomas that has been paired with economic analyses. Specifically, our management paradigm identifies patients who would benefit from surgery for asymptomatic peripheral nerve tumors, while providing cost-effective recommendations regarding clinical exams and serial imaging for such patients.
Keywords: Cost-effectiveness, cost-utility outcomes, health-related quality of life, peripheral nerve tumors, neurofibromas, schwannomas
EDITORIAL COMMENTS
Practicing good medicine no longer means simply optimizing a clinical outcome but also requires efficiency through application of cost-effective methods. The development and widespread use of screening techniques is significantly increasing the detection of pathological lesions including peripheral nerve sheath nerve tumors. Unfortunately, similar advances in predicting the subsequent behavior of such mass lesions has not yet occurred. Lacking a clinical “crystal ball,” the best clinicians can do is to develop evaluation and treatment algorithms that balance the costs and benefits of following such masses with serial clinical exams and imaging studies against the risks, benefits, and costs of surgical treatment. This is exactly what the article by Birk et al. attempts to do. Such algorithms must take into account many factors and uncertainties, which make them a work in progress. Although a step in the right direction, they are imperfect models in need of constant revision and improvement.
A MAJOR CLINICAL DILEMMA: EVALUATION AND TREATMENT OF NEWLY DIAGNOSED PERIPHERAL NERVE TUMORS
The most common nerve sheath tumors, neurofibromas and schwannomas, are benign well-encapsulated neoplasms that demonstrate a limited capacity for infiltration into surrounding tissues. Peripheral nerve schwannomas affect nearly 200,000 people in the United States, whereas neurofibromas affect 1 in 30000 Americans and are associated with NF1 and NF2 mutations.[
Analysis of the natural history and growth rate of these benign peripheral nerve tumors reveals that many do not grow, or may even stop growing naturally over time. Several studies show that anywhere from 58 to 69% of both peripheral and central vestibular schwannomas treated conservatively do not grow during observation periods.[
Given that the majority of peripheral nerve tumors are benign masses that grow slowly and may stop growing for very long periods of time, a major clinical dilemma arises when deciding how to appropriately manage asymptomatic peripheral nerve tumors. Widespread use of improved noninvasive imaging techniques has resulted in increased sensitivity in detecting nerve tumors. This has not been matched by improved specificity in determining which tumors will grow, become symptomatic, or even become malignant, versus which tumors will stop growing and remain asymptomatic. Just as increased rates of mammography and prostate-specific antigen screening have increased false positives for breast and prostate cancer treatment and led to unnecessary medical and surgical interventions,[
ESTABLISHING A COST-EFFECTIVE TREATMENT PARADIGM FOR MANAGEMENT OF PERIPHERAL NERVE TUMORS
Now that peripheral nerve tumors are more frequently identified with improved imaging techniques, the question arises as to how to most effectively distinguish which tumors will grow and cause symptoms from those that do not grow or grow at such a slow rate that the likelihood of symptom development is very small. Recent research in the realm of asymptomatic vestibular schwannomas supports a “wait-and-scan policy” with MRI scans performed annually for vestibular schwannoma surveillance.[
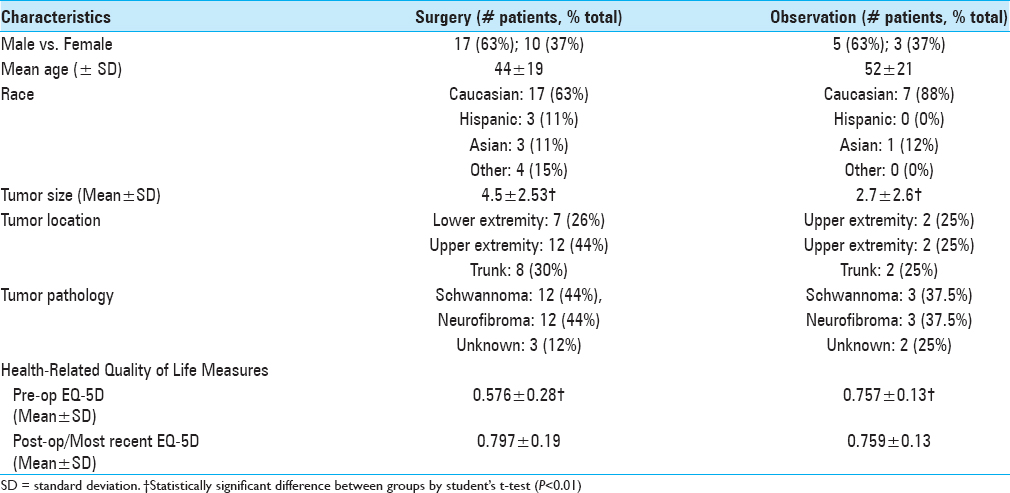
To develop our paradigm, we first performed a retrospective review of the demographic and clinical characteristics, outcomes, and management costs for 35 peripheral nerve tumors patients who were either observed (n = 8) or surgically treated (n = 27) by a single surgeon (M.K.) at the University of California at San Francisco (UCSF) from January 2012 to December 2013 [
Health-related quality of life (HRQoL) was assessed in both cohorts of patients via pre and postoperative EQ-5D, a well-validated instrument for measuring generic health status on a scale of 0 to 1. On average, the surgical patients reported a significantly lower preoperative EQ-5D score as compared to the observation patients (0.576 vs. 0.757; P < 0.01). Following surgical resection, patients’ EQ-5D improved considerably, and their postoperative EQ-5D score was very similar to the most recent EQ-5D scores of patients who were observed (0.797 vs. 0.759, P = 0.298). Observation patients remained at similar EQ-5D scores at the beginning and end of the observation period (0.757 vs. 0.759).
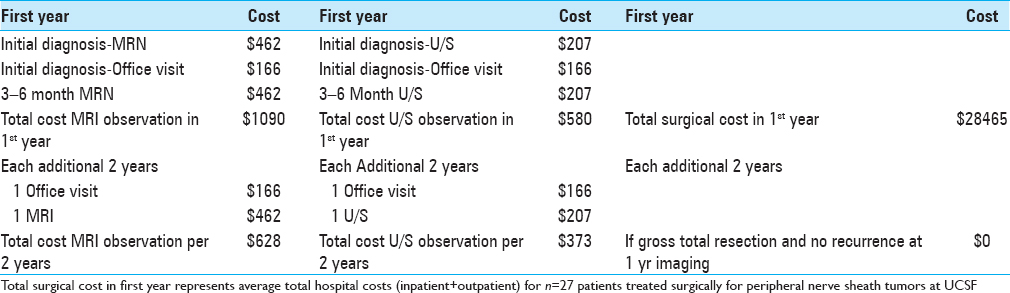
We then performed a cost analysis of our observed versus surgically treated peripheral nerve tumor patients. For observed patients, we estimated the total cost for observation, allowing for either magnetic resonance neurograms (MRN) or ultrasound (U/S) imaging 3–6 months after the initial diagnostic scan, and then every 2 years afterwards if the tumor remained stable [
For surgical patients, the actual total inpatient and outpatient hospital costs were also determined from the UCSF hospital cost accounting database. The average total cost for treating peripheral nerve tumors surgically was $28465 [
Next, we performed a cost-effectiveness analysis for a patient diagnosed with a peripheral nerve tumor at age 45 (the average age of our total patient cohort). We calculated quality-adjusted life years (QALYS) using our EQ-5D health utilities measure, and used a standard discounting factor of 0.03.
CONCLUSION
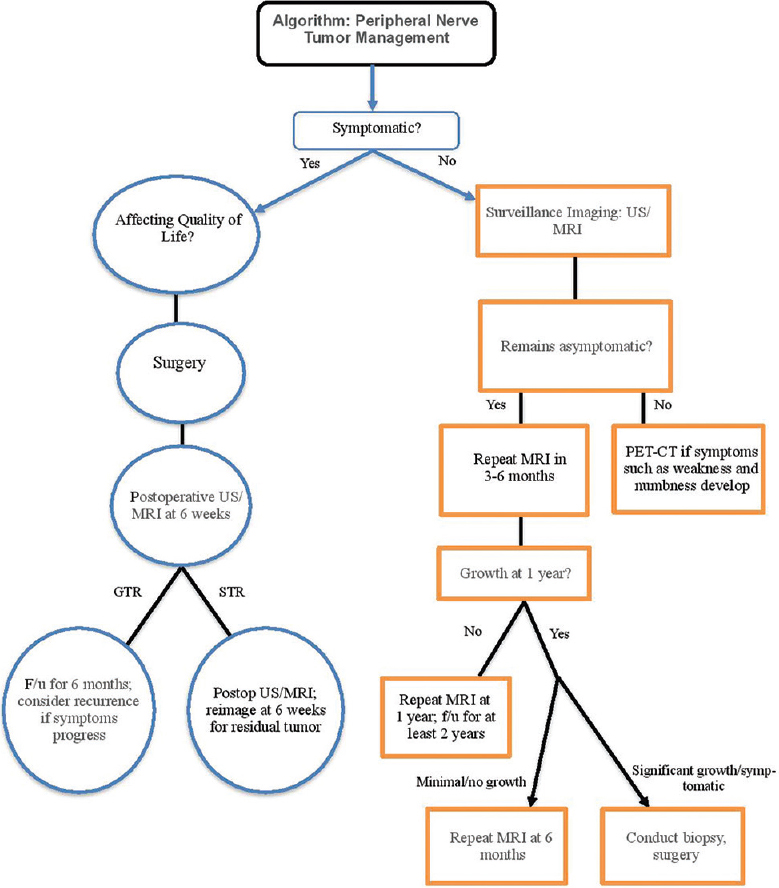
In summary, we propose the following paradigm [
An important limitation of our study is our small dataset, with the number of surgically treated patients being over three times larger than our medically treated patient cohort. This reflects the referral patterns to the neurosurgeon M.K. at a tertiary care facility. Another limitation is our study design, which is retrospective in nature. A more robust study would be prospective in nature and compare patients with peripheral nerve tumors that had equipoise, i.e. could be treated either medically or surgically. The lower EQ-5D and larger tumor size of our surgical group suggests that our patient cohorts were not identical in this small retrospective study.
Nevertheless, we find that surgery can be cost-effective in our patient cohort. We use our own institution's cost data, as well as literature review and clinical experience, to propose a management guideline for newly diagnosed peripheral nerve sheath tumors, which we believe will be useful to the practicing neurosurgeon.
References
1. Al Sanosi A, Fagan PA, Biggs ND. Conservative management of acoustic neuroma. Skull Base. 2006. 16: 95-100
2. DeWeerdt S. Prognosis: Proportionate response. Nature. 2015. 528: S124-5
3. Kliot T, Ince Y, Tihan T, Wilson M, Kliot M. To grow or not to grow, That is the question. Sure Neurol Int. 2013. 4: S407-10
4. Luetje CM. Spontaneous involution of acoustic tumors. Am J Otol. 2000. 21: 393-8
5. Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: Alternative approaches. Bull World Health Organ. 2015. 93: 118-24
6. Nutik SL, Babb MJ. Determinants of tumor size and growth in vestibular schwannomas. J Neurosurg. 2001. 94: 922-6
7. Patnaik U, Prasad SC, Tutar H, Giannuzzi AL, Russo A, Sanna M. The long-term outcomes of wait-and-scan and the role of radiotherapy in the management of vestibular schwannomas. Otol Neurotol. 2015. 36: 638-46
8. Rosenberg SI. Natural history of acoustic neuromas. Laryngoscope. 2000. 110: 497-508
9. Silverstein H, McDaniel A, Morrell H, Wazen J. Conservative management of acoustic neuroma in the elderly patient. Laryngoscope. 1985. 95: 766-70
10. Sohn E. Screening: Don’t look now. Nature. 2015. 527: S118-9
11. Sughrue ME, Kaur R, Rutkowski MJ, Kane AJ, Kaur G, Yang I. Extent of resection and the long-term durability of vestibular schwannoma surgery. J Neurosurg. 2011. 114: 1218-23
12. Sughrue ME, Yang I, Aranda D, Lobo K, Pitts LH, Cheung SW. The natural history of untreated sporadic vestibular schwannomas: A comprehensive review of hearing outcomes. J Neurosurg. 2010. 112: 163-7
13. Swan IR. Is early management of acoustic neuroma important?. J R Soc Med. 2000. 93: 614-7
14. Theodosopoulos PV, Pensak ML. Contemporary management of acoustic neuromas. Laryngoscope. 2011. 121: 1133-7
15. Tschudi DC, Linder TW, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol. 2000. 21: 722-8
16. Wazen J, Silverstein H, Norrell H, Besse B. Preoperative and postoperative growth rates in acoustic neuromas documented with CT scanning. Otolaryngol Head Neck Surg. 1985. 93: 151-5
17. Zygourakis CC, Oh T, Sun MZ, Barani I, Kahn JG, Parsa AT. Surgery is cost-effective treatment for young patients with vestibular schwannomas: Decision tree modeling of surgery, radiation, and observation. Neurosurg Focus. 2014. 37: E8-