- Professor of Clinical Neurosurgery, School of Medicine, State University of New York at Stony Brook, New York, United States.
DOI:10.25259/SNI_54_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Diagnosis and Safe Excision of Lumbar Synovial Cysts and Accompanying Pathology: A Perspective. 28-Feb-2020;11:33
How to cite this URL: Nancy E. Epstein. Diagnosis and Safe Excision of Lumbar Synovial Cysts and Accompanying Pathology: A Perspective. 28-Feb-2020;11:33. Available from: https://surgicalneurologyint.com/surgicalint-articles/9881/
Abstract
Background:Lumbar synovial cysts are often not sufficiently diagnosed prior to spine surgery. Utilizing both MR and CT studies is critical for recognizing the full extent/severity of these lesions.
Methods:In patients with chronic, acute, or subacute lumbar disease, obtaining both MR and CT studies is critical to correctly diagnose; disc disease, hypertrophy/ossification of the yellow ligament (OYL), stenosis, with/without degenerative spondylolisthesis, and/or synovial cysts (SC).
Results:MR T2 weighted images directly demonstrate hyperintensity within a SC. They initially cause lateral recess/caudad nerve root and/foraminal compromise, with larger extrusions causing significant lateral thecal sac, and far lateral/superior cephalad root compromise. CT 2 mm cuts often better demonstrate mid-vertebral level compression of cephalad nerve roots with/without SC calcification, along with the extent of mid-vertebral stenosis, hypertrophy/OYL, and DS. When CT studies directly document SC calcification, it alerts the surgeon to the increased potential risk of creating a cerebrospinal fluid fistula with full SC excision, and should prompt the adoption of alternative measures such as decompression/partial removal. Most critically, surgery for synovial cysts often warrants a 2-level laminectomy for fuller visualization of the cephalad and caudad nerve roots, and clearer differentiation of neural tissues from the large fibrotic SC capsule, to effect safer removal.
Conclusions:Preoperatively, establishing the full cephalad and cauda extent of lumbar synovial cysts with both MR and CT studies is critical. Anticipation and better visualization of the foraminal/far lateral and superior extent of these lesions often warrants more extensive multilevel laminectomies for thecal sac and both cephalad and caudad root decompression.
Keywords: Computed tomography, Degenerative spondylolithesis, Magnetic resonance, Multilevel laminectomy, Noninstrumented fusion, Rare instrumented fusion, Safe surgery, Synovial Cysts: Lumbar stenosis
INTRODUCTION
Both MR and CT studies are essential to preoperatively document the full extent of lumbar stenosis, hypertrophy/ossification of the yellow ligament (OYL), with/without degenerative spondylolisthesis (DS), and synovial Cysts (SC) [
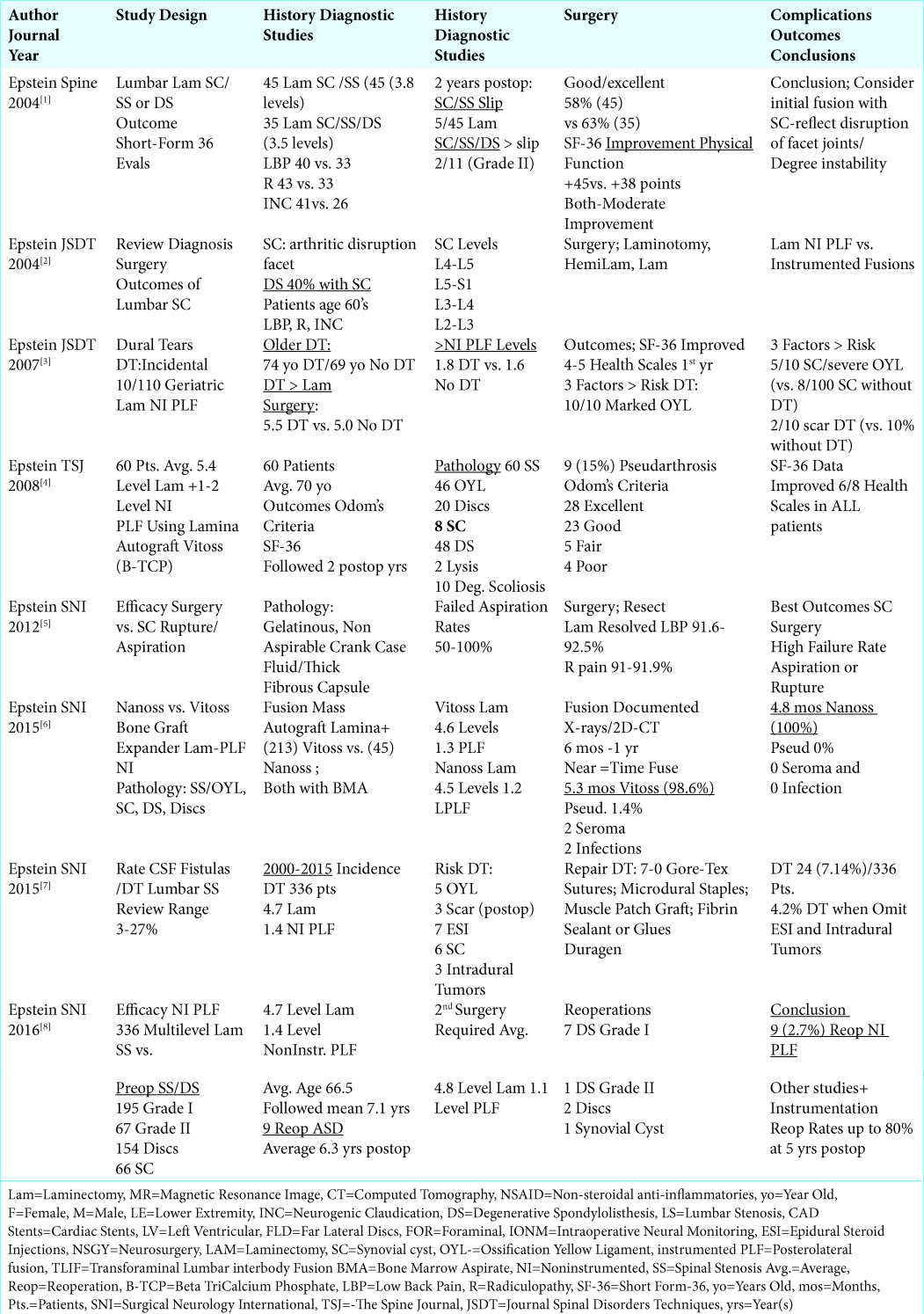
Figure 1:
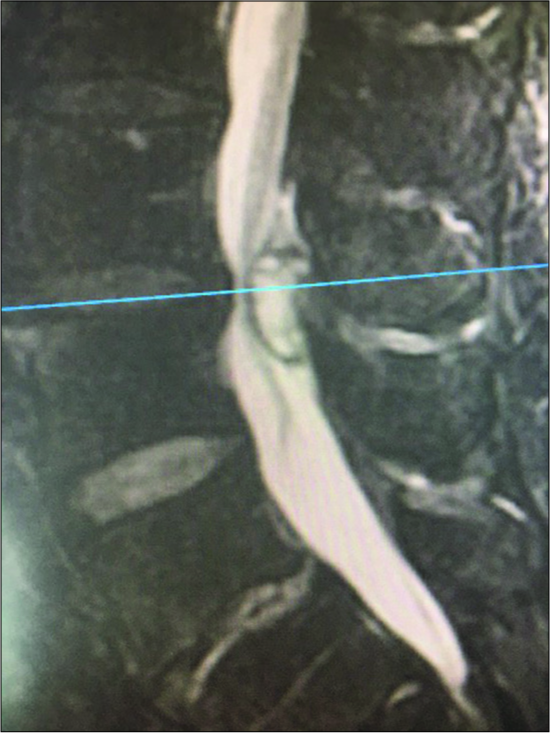
T2 axial weighted MR demonstrating extrusion of a synovial cyst originating from the right facet joint (also containing a high T2 weighted signal) at the disc space level. This resulted in marked right root and severe dorsolateral thecal sac/cauda equina compression. Also note fluid in both facet joints.
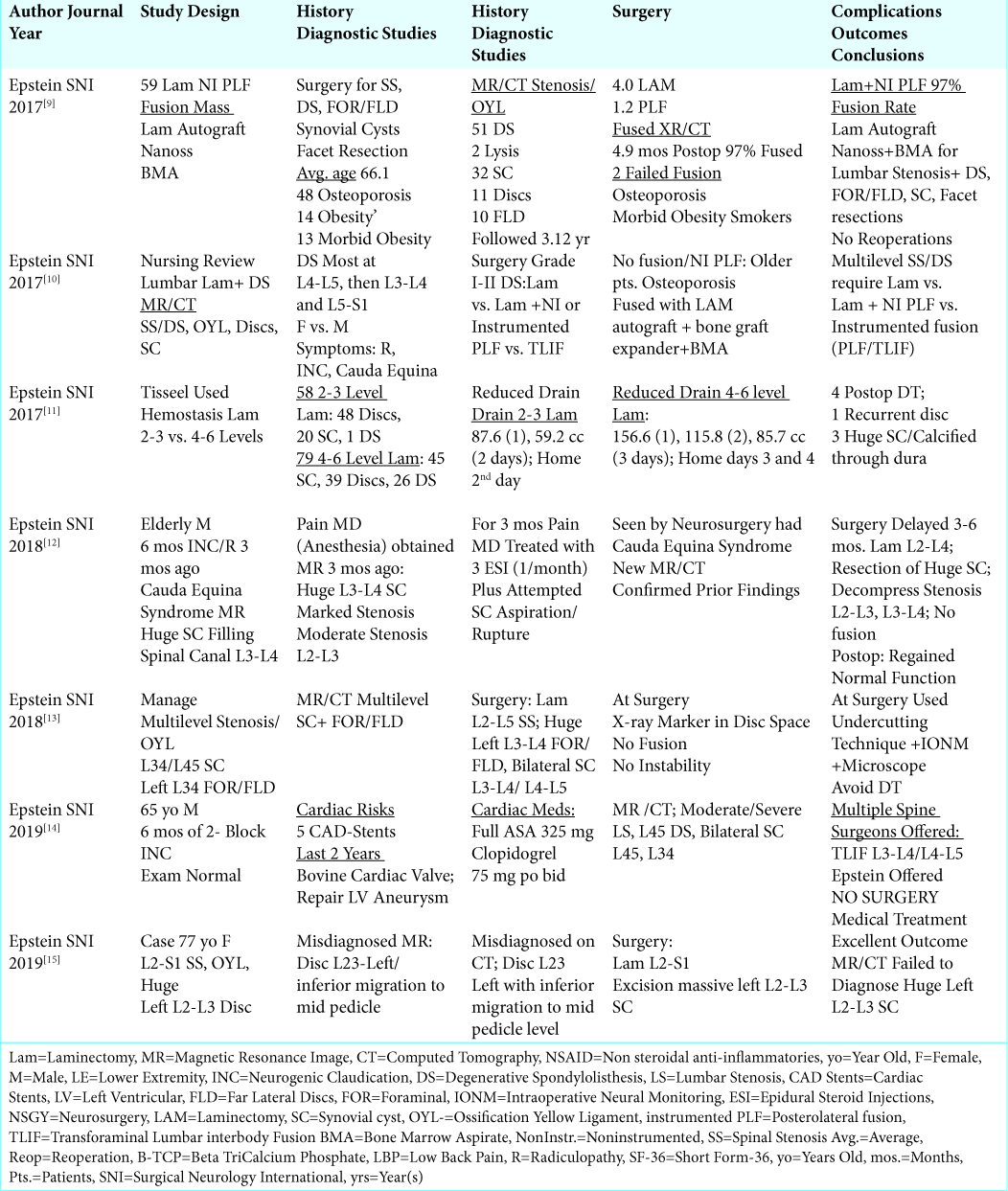
Figure 2:
Large central synovial cyst extrusion resulting in diffuse bilateral thecal sac/cauda equina compression. Note on this study there are hyperintense signals within both facet joints, and it is difficult to determine whether this cyst originated from the right or left facets. Further, observe the hypertrophy/ossification (hypointense signal) of the yellow ligament.
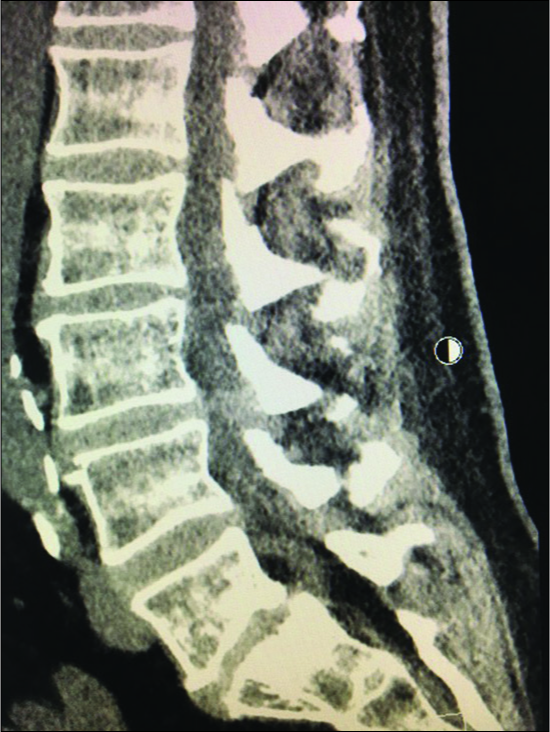
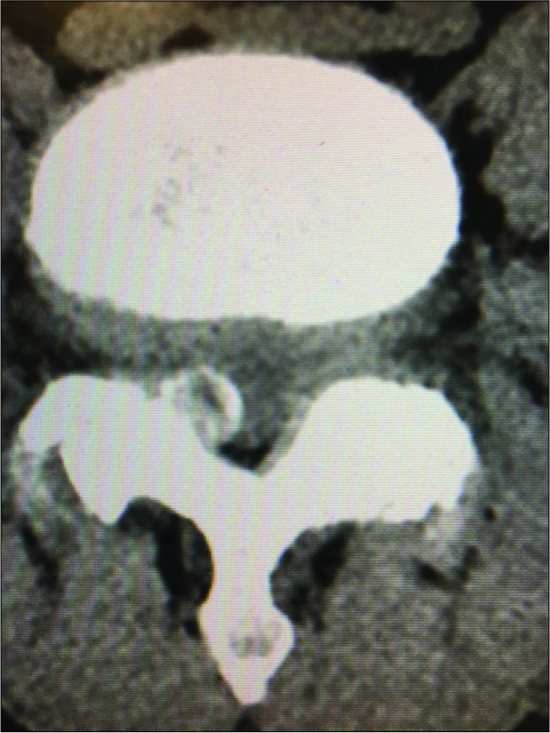
Figure 4:
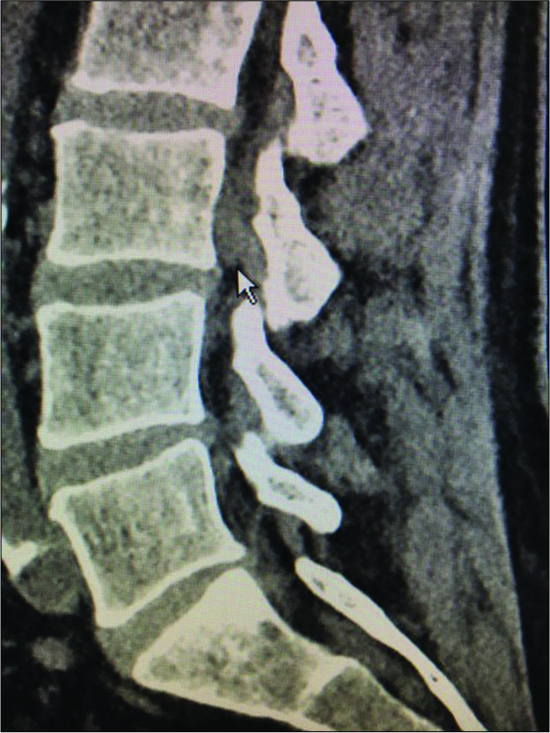
Parasagittal CT at the L3-L4 level (same patient as
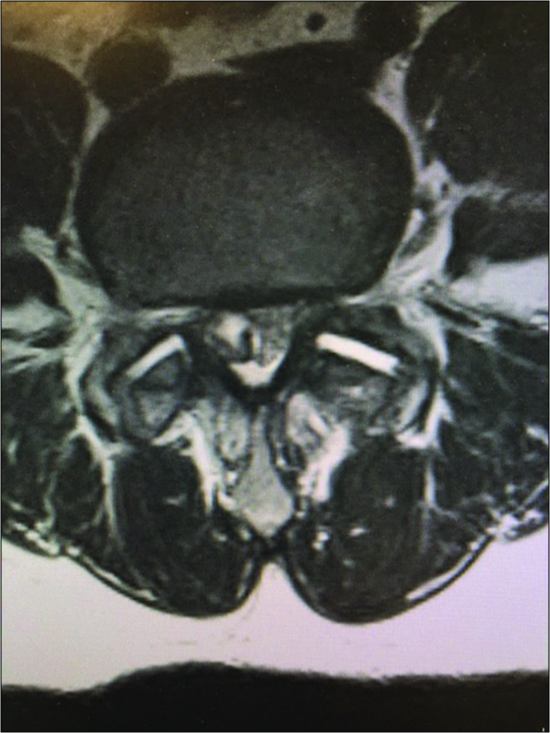
Figure 6:
In the same patient as
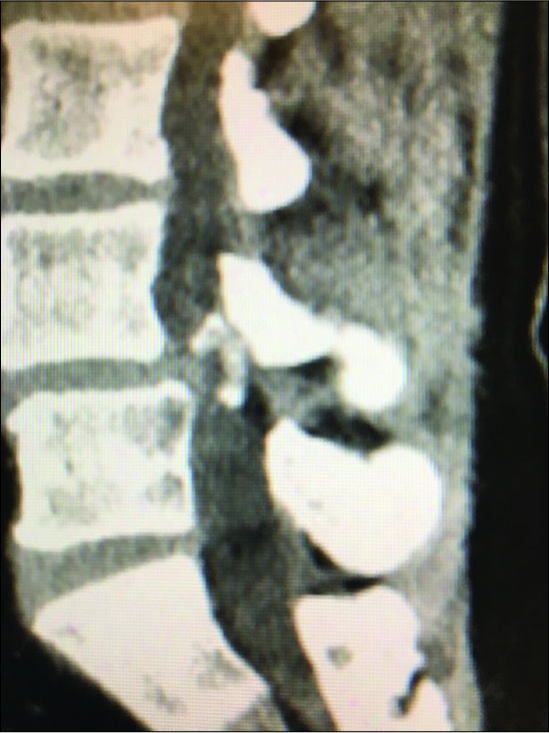
Figure 9:
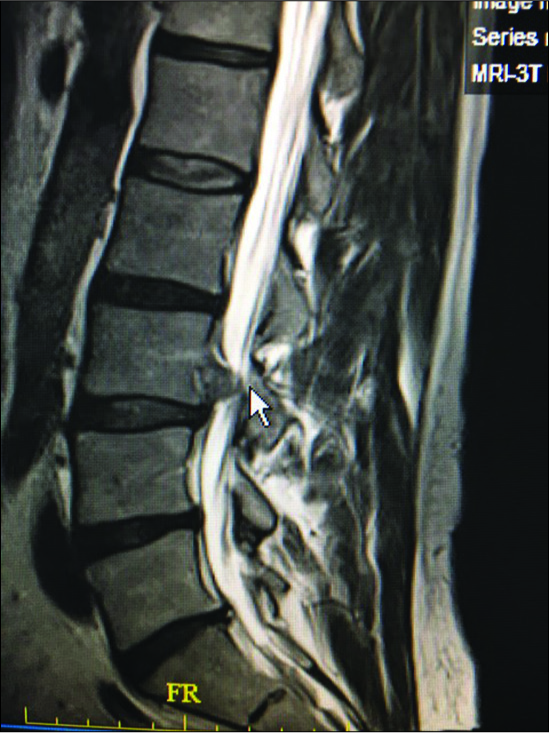
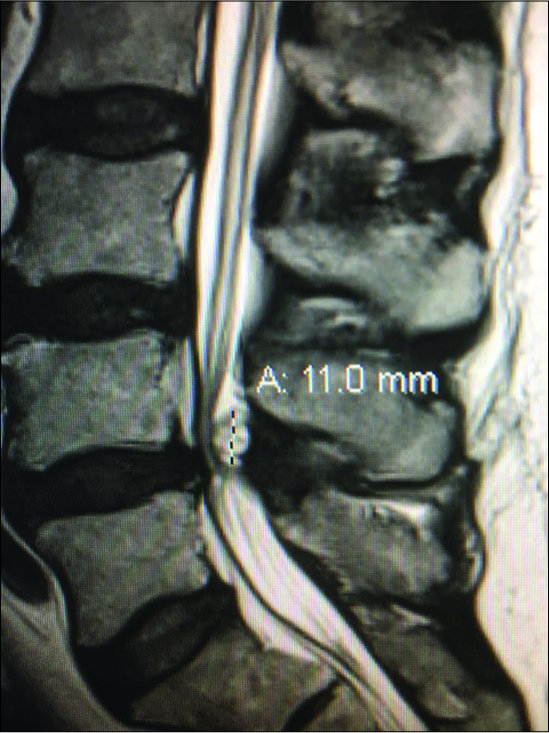
Large dorsolateral synovial cyst on a T2 parasagittal MR study measuring 11 mm in the cephalad-caudad dimension. Notes its origin from the L4-5 level with cephalad extension beyond the mid-vertebral level. The cyst itself exhibits multiple inhomogeneous densities indicative of the variegated solid contents, and minimal actual crank-case fluid.
Figure 10:
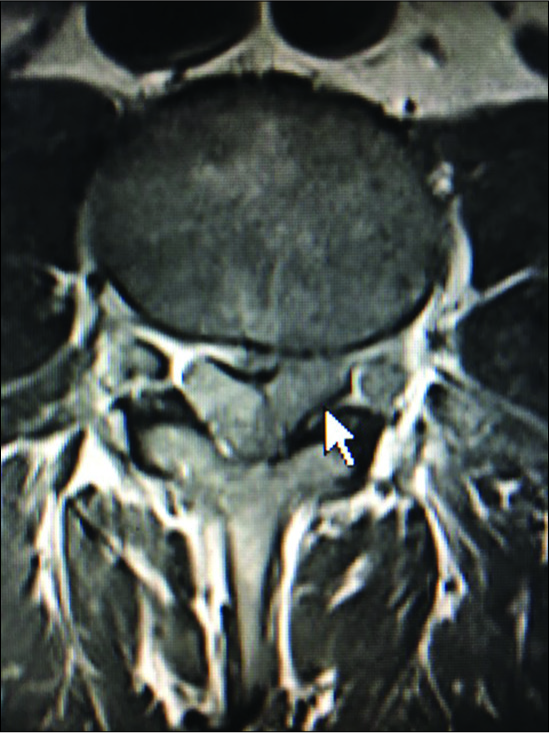
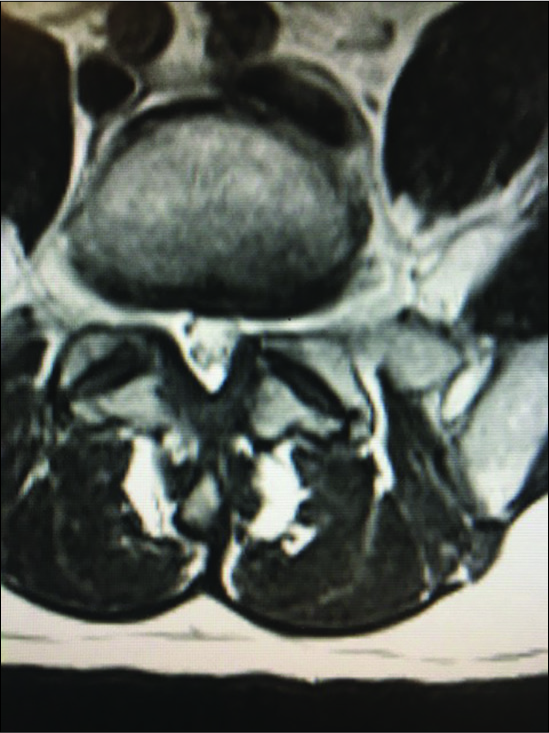
On the axial T2 weighted MR scan there is a mildly hyperintense structure on the left and the right sides in continuity with the facet joint indicating likely bilateral synovial cysts rather than just hypertrophy of the yellow ligament. On the left, this results in mild/moderate compromise of the nerve root in the lateral recess.
Figure 11:
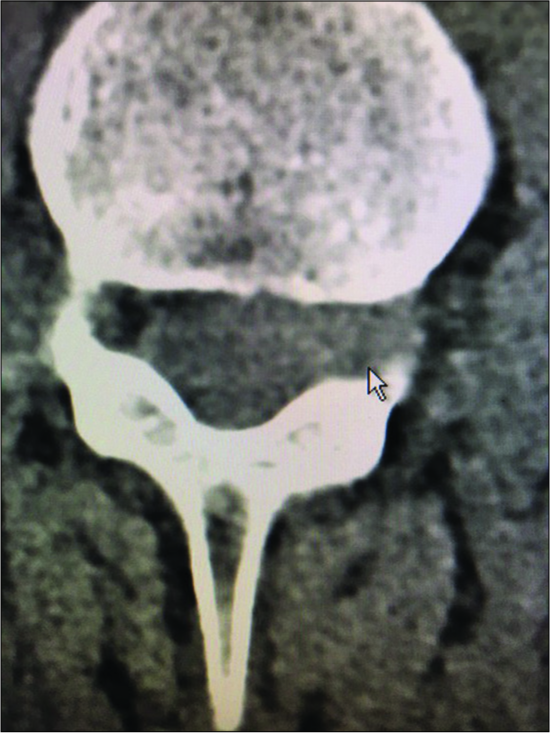
On this axial non-contrast CT scan, at the L4-L5 level, the capsule of the synovial cyst on the right side of the spinal canal is ossified with central ihomogeneous and hypodense components. Such synovial cysts are often comprised of multiple layers of dense fibrous ossified capsule, and may contain minimal amounts of actual fluid (e.g. crank case fluid or more typically hypodense soft tissue).
Figure 12:
The same patient as in
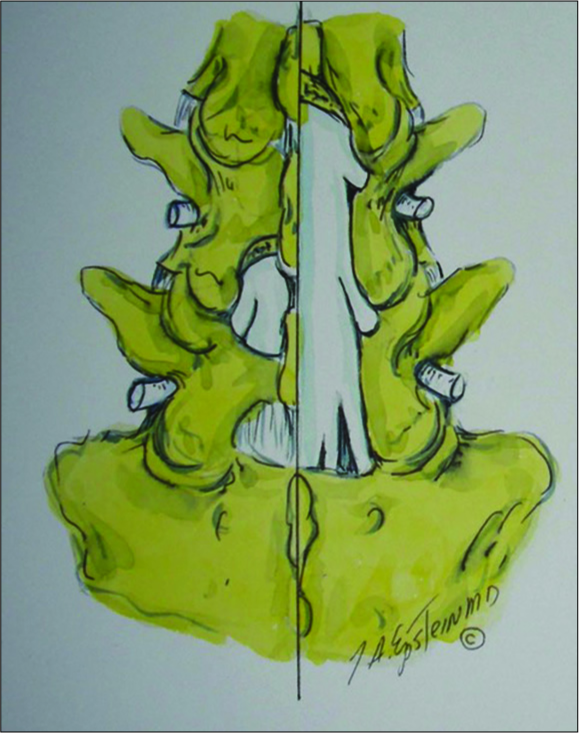
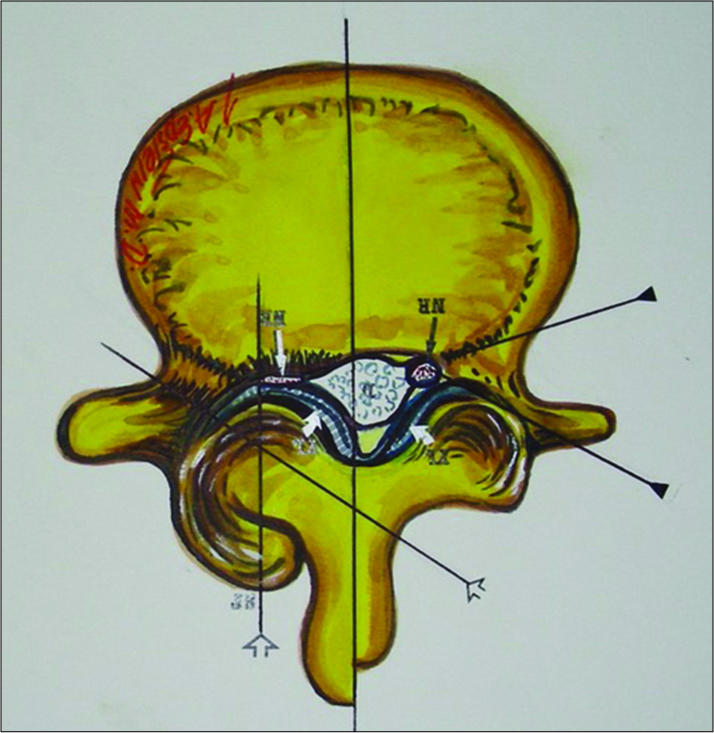
Figure 14:
In this illustration by Joseph A. Epstein, M.D., the right side shows a multilevel hemilaminectomy that would potentially be used for excision of an L4-L5 synovial cyst (e.g. with lateral, foraminal, far lateral superior extension compressing the L4, and L5 roots), as long as there is also significant stenosis L3-S1 requiring decompression.
Figure 15:
This axial illustration by Joseph A. Epstein, M.D. demonstrates marked right-sided facet hypertrophy with hypertrophy of the yellow ligament resulting (short thick arrows) in use severe compression of the nerve root (thin white arrow) in the lateral recess. In many similar cases. There is also an accompanying component of synovial cyst extrusion as well.
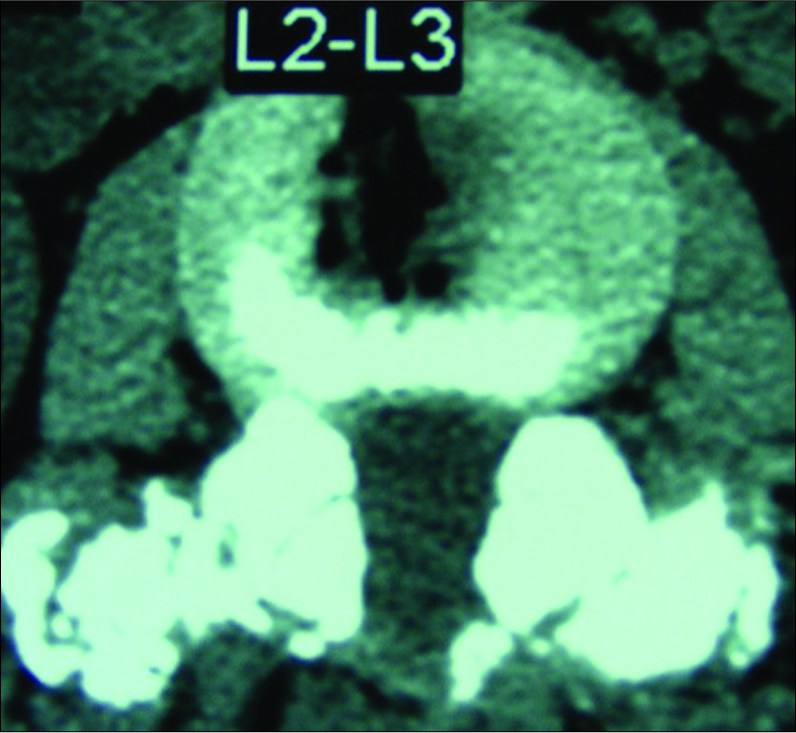
Figure 16:
In some cases, particularly where there is degenerative spondylolisthesis at the level of a synovial cyst extrusion, one might consider performing a non-instrumented posterolateral fusion as shown here at the L2-L3 level. Note the marked fusion mass consisting of autologous bone combined with Vitoss/BMA placed posterolaterally and bilaterally over the transverse processes at the L2-L3 level.
Anatomy of Synovial Cysts and Radiological Appearance on MR
Pathoanatomically, synovial cysts are comprised of cystic dilatations of synovial sheaths that have directly extruded from the overlying facet joints into the spinal canal due to disruption of the facet joint capsule [
Radiological Diagnosis of Synovial Cysts with MR
MR studies are usually the first and best examinations performed to identify lumbar synovial cyst extrusions in conjunction with lumbar stenosis, OYL, with/without DS (e.g. some cite a 40% incidence of DS with SC) [
Limitations of MR
MR axial 5 mm thick sections are focused at the disc space levels. Typically, one images is taken above, another at, and the final section just below the interspace. A limitation of axial MR studies is that they may mis/underrepresent mid-level vertebral pathology [
Utility of CT Examinations in Diagnosing Lumbar Stenosis, OYL, DS, and SC
However, the non-contrast CT 2 mm axial and sagittal scans may better demonstrate mid-level vertebral disease along with stenosis, OYL, DS, spondylolisthesis/lysis, and SC, especially when considering surgery [
Case: When MR/CT Examinations are Interpreted as Disc, Missing Synovial Cyst
A 77-year-old female presented with several weeks of increasing left lower extremity neurogenic claudication/ radiculopathy accompanied by proximal left leg weakness (2/5), and numbness (pin loss L2-L3 distributions) [
Pain Management (PM) Specialists’ Fail to Manage Lumbar Synovial Cysts
The 2012, Epstein and Baisden reviewed the SC literature; it showed 50-100% failure rates for PM attempts at synovial cyst “rupture/aspiration” under fluoroscopic/ CT-guidance while performing ESI (e.g. ESI with no documented long-term efficacy) [
Case; PM Fail to “Rupture/Aspirate” Synovial Cyst Critically Delaying Surgery
PM followed an elderly male (e.g. >75) for over 6 months with progressive low back pain, neurogenic claudication, and radiculopathy [
More Extensive “Open” Surgery for Safe Synovial Cyst Excision
A 2-level decompression simultaneously provides safe excision of SC, along with decompression of bilateral stenosis/ OYL with/without DS. This is typically warranted due to SC’ foraminal, far lateral, and superior extension [
Undercutting Laminectomy/Decompression of Stenosis/ OYL with/without DS/SC
Using an angled Kerrison rongeur, performing an undercutting laminectomy for SC/stenosis/OYL/DS decompression/excision involves working under an operating microscope from the opposite side of the table; further the patient is rotated/angled away from the surgeon. This technique largely provides adequate exposure while facilitating preservation of the overlying laminae/facets, use; maintaining stability, and averting fusion. It is essential to attain sufficient exposure to avoid the minimally invasive (MI) (e.g. including TLIF) increased complications that too often include; inadvertent neural/dural injury, and incomplete cyst removal.
Documentation of Location/Level of Synovial Cyst Removal with 2 X-rays
When opening at the level of a SC, the lesion may not be readily apparent. It is imperative to obtain two intraoperative radiographs to confirm the correct level; first, a cross table lateral X-ray with a clamp on the correct interspinous ligament, and second, placing a Penfield elevator in the correct disc interspace (e.g. not just in the canal). This is how to both confirm the level of the SC extrusion, as well as to avoid wrong-level surgery.
Surgery for Multilevel Stenosis/OYL, Synovial Cysts (SC), and a Foraminal (FOR)/Far Lateral Disc (FLD)
A patient (2018) presented with L2-L5 stenosis/OYL, a left L3-L4 foraminal (FOR)/far lateral disc (FLD), and bilateral L3-L4/L4-L5 synovial cysts [
Fusion vs. No Fusion for Lumbar Synovial Cysts with Other Pathology
Fusion options following lumbar decompressions include; non-instrumented posterolateral fusions (PLF) vs. instrumented PLF, or rarely TLIF [
2004: Recommended Possible Fusion for SC with Lumbar Stenosis/OYL with/without DS
In a 2004 study, Epstein evaluated the outcomes/ frequencies of instability 2 years after multilevel laminectomies for stenosis/OYL (average 3.8 levels: 45 patients) with SC vs. multilevel laminectomies for stenosis/OYL (average 3.5 levels: 35 patients) with synovial cysts (SC), and degenerative spondylolisthesis (DS) [
2017: No Fusion Typically Required for Laminectomy Stenosis/OYL with/without SC/DS
In 2017, Epstein documented no need for fusion following 2-3 level (58 patients) vs. 4-6 level (79 patients) lumbar laminectomies for spinal stenosis/OYL with/without SC, and DS. [
Tisseel: Promotes Hemostasis Following Lumbar Laminectomies
Tisseel (Baxter International Inc., Westlake Village, CA, USA), a fibrin sealant, was also routinely used to minimize postoperative bleeding, facilitate hemostasis following the 2-3 (58 patients) vs. 4-6 (79 patients) level lumbar laminectomies for stenosis/OYL with/without SC, and DS [
Noninstrumented PLF for Laminectomy with Stenosis/ OYL with/without SC/DS Using Different Bone Graft Expanders
Non-Instrumented PLF Rates Using Vitoss
Select patients undergoing decompressive lumbar laminectomies for stenosis/OYL, foraminal/far lateral discs, synovial cysts, and DS requiring full facetectomy with major comorbidities may warrant noninstrumented posterolateral fusions (PLF) [
In a second study in 2016, 336 patients underwent multilevel laminectomies with noninstrumented multilevel fusions using Vitoss/B-TCP/lamina autograft/BMA [
Noninstrumented Fusion Rates for Vitoss vs. Nanoss
In 2015, Epstein compared the relative efficacy of Vitoss (213 patients) vs. Nanoss (45 patients; Regeneration Technologies Corporation: RTI, Alachua, FL, USA) using comparable noninstrumented PLF techniques [
Noninstrumented Fusion Using Nanoss Alone
Subsequently, Epstein (2017) assessed 59 patients undergoing average 4.0 level laminectomies and average 1.2 level noninstrumented fusions for stenosis/OYL, DS (51 patients), synovial cysts (32 patients), and other pathology using Nanoss (RTI Surgical Alachua, FL, and USA) with lamina autograft/BMA [
Frequency of Dural Tears (DT) with Lumbar Synovial Cyst Surgery
The presence of synovial cysts increases the risk of intraoperative dural tears (DT) (range 3-27%) during multilevel lumbar laminectomies with/without noninstrumented vs. instrumented lumbar fusions [
Recommended Repair Techniques for DT
Recommended repair techniques for DT include using 7-0 Gore-Tex (Newark, Delaware, USA) sutures (e.g. where the needle is smaller than the suture itself, thus filling the needle holes), micro-dural staples (1.4 mm), muscle patch/ bovine pericardial grafts, application of Duragen (Integra LifeSciences, Hawthorne, NY, USA), and use of a fibrin sealant (e.g. safely used Tisseel; Baxter International Inc., Westlake Village, CA, USA).
Intraoperative Neural Monitoring; Critical Adjunct to Lumbar Surgery
Extremely useful in performing multilevel laminectomies with/without non instrumented fusions is intraoperative neural monitoring. It typically consists of electromyography (EMG: some also including sphincteric monitoring), and somatosensory evoked potential monitoring (SEP); the EMG will signal root and/or sphincteri compromise, while SEP may indicate significant thecal sac/cauda equina compression. Anesthesia’s utilization of TIVA (total intravenous anesthesia) best protects the quality of these continuous EMG/SEP recordings [
When to Say No to Surgery Due to Major Comorbidities
Not all patients with significant MR/CT documented spinal stenosis, OYL with/without synovial cysts and/or DS are appropriate surgical candidates. Major comorbidities must be carefully taken into consideration, as the risks of surgery may outweigh the benefits. The following case report illustrates this point. A 65-year-old male presented with 6 months of 2-block neurogenic claudication, but a normal neurological exam [
CONCLUSIONS
Lumbar synovial cysts should be more fully diagnosed preoperatively utilizing both MR and CT to demonstrate foraminal/far lateral, and superior compression of the cephalad, cauda nerve roots, and lateral thecal sac [
Declaration of patient consent
Not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Epstein NE. Lumbar laminectomy for the resection of synovial cysts and coexisting lumbar spinal stenosis or degenerative spondylolisthesis: an outcome study. Spine Iphila Pa 1976). 2004. 1:29: 1049-55
2. Epstein NE. Lumbar synovial cysts: a review of diagnosis, surgical management, and outcome assessment. J Spinal Disord Tech. 2004. 17: 321-5
3. Epstein NE. The frequency and etiology of intraoperative dural tears in 110 predominantly geriatric patients undergoing multilevel laminectomy with noninstrumented fusions. J Spinal Disord Tech. 2007. 20: 380-6
4. Epstein NE. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008. 8: 882-7
5. Epstein NE, Baisden J. The diagnosis and management of synovial cysts: Efficacy of surgery versus cyst aspiration. Surg Neurol Int. 2012. 3: S157-66
6. Epstein NE. Preliminary study showing safety/efficacy of nanoss bioactive versus vitoss as bone graft expanders for lumbar noninstrumented fusions. Surg Neurol Int. 2015. 6: S318-22
7. Epstein NE. Incidence and management of cerebrospinal fluid fistulas in 336 multilevel laminectomies with noninstrumented fusions. Surg Neurol Int. 2015. 6: S463-8
8. Epstein NE. Low reoperation rate following 336 multilevel lumbar laminectomies with noninstrumented fusions. Surg Neurol Int. 2016. 7: S331-6
9. Epstein NE. High lumbar noninstrumented fusion rates using lamina autograft and Nanoss/bone marrow aspirate. Surg Neurol Int. 2017. 8: 153-
10. Epstein NE, Hollingsworth RD. Nursing review of diagnosis and treatment of lumbar degenerative spondylolisthesis. Surg Neurol Int. 2017. 8: 246-
11. Epstein NE. Tisseel’s impact on hemostasis for 2-3 and 4-6-level lumbar laminectomies. Surg Neurol Int. 2017. 8: 299-
12. Epstein NE. Spinal case of the month with short perspective: How would you treat this L3-L4 synovial cyst?. Surg Neurol Int. 2018. 9: 56-
13. Epstein NE. Case presentation and short perspective on management of foraminal/far lateral discs and stenosis. Surg Neurol Int. 2018. 9: 87-
14. Epstein NE. Avoiding inappropriate spine surgery in a patient with major cardiac comorbidities. Surg Neurol Int. 2019. 10: 44-
15. Epstein NE. Case of the Week: Preoperative MR/CT Diagnosis of Left L2-L3 Disc Surgically Documented As Massive Synovial Cyst. Surg Neurol Int. 2019. 10: 168-






Robert Anthony
Posted November 14, 2023, 2:12 pm
Dr. Nancy Epstein,
As a 68-yr male patient newly diagnosed [MR only] with a right-side L5 synovial cyst, and suffering moderate to intense buttock and ankle pain over 3+ months (preceded by ~12 months of ‘random’ back pain), I find your published work vital to understanding the need for MR & CT image analysis prior to considering surgery.
Having also read references 5 & 12, I am putting my PM’s recommendation for ESI/SC aspiration/ rupture “ON HOLD” until I seek a 2nd opinion, hopefully with 2-mm CT imaging to preclude dural adherence. To be blunt, I sensed an over-confidence from my PM when I asked about permanent vs. temporal pain relief; your documented statistic of 50-100% failure with cyst aspiration certainly beats the smirk and no quantitative answer that I received from the PM.
As an aerospace mechanical engineer, I value your approach to addressing the root cause of lumbar ailments by utilizing multiple imaging methods along with statistical case history when diagnosing and determining treatment. From your published works and my 20+ years of casual experience using CT imaging for space structure defect analysis, I was able to determine the location, size and density of my L5 cyst using the DICOM viewer. I hope to gain more insight when meeting a U of P spine surgeon next week.
I am most grateful for your passion and pursuit of excellence in the application of spine surgery. I trust your published works will continue to inspire current and future students beyond your imagination.
Best regards,
Bob Anthony