- Clinical Professor of Neurosurgery, School of Medicine, State University of New York at Stony Brook.
DOI:10.25259/SNI_294_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy Epstein. Diagnosis, and Treatment of Cervical Epidural Abscess and/or Cervical Vertebral Osteomyelitis with or without Retropharyngeal Abscess; A Review. 20-Jun-2020;11:160
How to cite this URL: Nancy Epstein. Diagnosis, and Treatment of Cervical Epidural Abscess and/or Cervical Vertebral Osteomyelitis with or without Retropharyngeal Abscess; A Review. 20-Jun-2020;11:160. Available from: https://surgicalneurologyint.com/surgicalint-articles/10095/
Abstract
Background: Every year approximately 19.6 patients/100,000 per year are admitted to hospitals with spinal epidural abscesses (CSEA), 7.4/100,000 have vertebral osteomyelitis (VO)/100,000/year, while 4.1/100.000 children/year have cervical retropharyngeal abscesses (RPA) (i.e., data insufficient for adults).
Methods: Here we evaluated 11 individual case studies, 6 multiple patient series, and looked at 9 general review articles focusing on CSEA, and/or VO, with/without RPA.
Results: Of the 11 case studies involving 15 patients, 14 had cervical spinal epidural abscesses (CSEA: 10 CSEA/ VO/RPA, 2 CSEA/VO, 1 CSEA/TSEA, 1 CSEA/ TSEA/LSEA), 13 had cervical osteomyelitis (VO: 11 VO/CSEA, 2 VO/RPA), and 12 had cervical retropharyngeal abscesses (RPA: 10 RPA/CSEA/VO, 2 RPA/VO alone). When patients were treated surgically, they required 12 anterior, and 2 posterior approaches; 1 patient required no surgery. In the 6 larger cervical series involving 355 patients, 4 series involved CSEA (3 CSEA, 1 CSEA/VO), and 2 seires had cervical VO. Primary surgery was performed in 298 patients, while 57 were initially managed medically; 24 of these latter patients failed non-surgical therapy, and required delayed cervical surgery. Notably, all 17 clinical studies advocated early surgery where clinically appropriate for varying combinations of CSEA and/or VO with or without RPA. The 8 final articles reviewed all-levels of SEA and or VO, while also providing additional unique information regarding RPA.
Conclusion: We analyzed 11 case studies and 6 multiple case series regarding the diagnosis and treatment of combinations of cervical CSEA, and/or VO with or without RPA. We also reviewed 8 articles on the evaluation/ management of all-level SEAs and/or VOs, along with the unique features of RPAs.
Keywords: Cervical spine epidural abscesses, How to recognize failure of medical management, Retropharyngeal abscesses, Success of early surgery where appropriate for CSEA and/or VO with/without RPA, Vertebral osteomyelitis
INTRODUCTION
Every year approximately 19.6 patients/100,000 are admitted to hospitals with spinal epidural abscesses (CSEA), 7.4/100,000 have vertebral osteomyelitis (VO) /100,000, and 4.1/100,000 children have cervical retropharyngeal abscesses (RPA) (i.e. data insufficient for adults).[
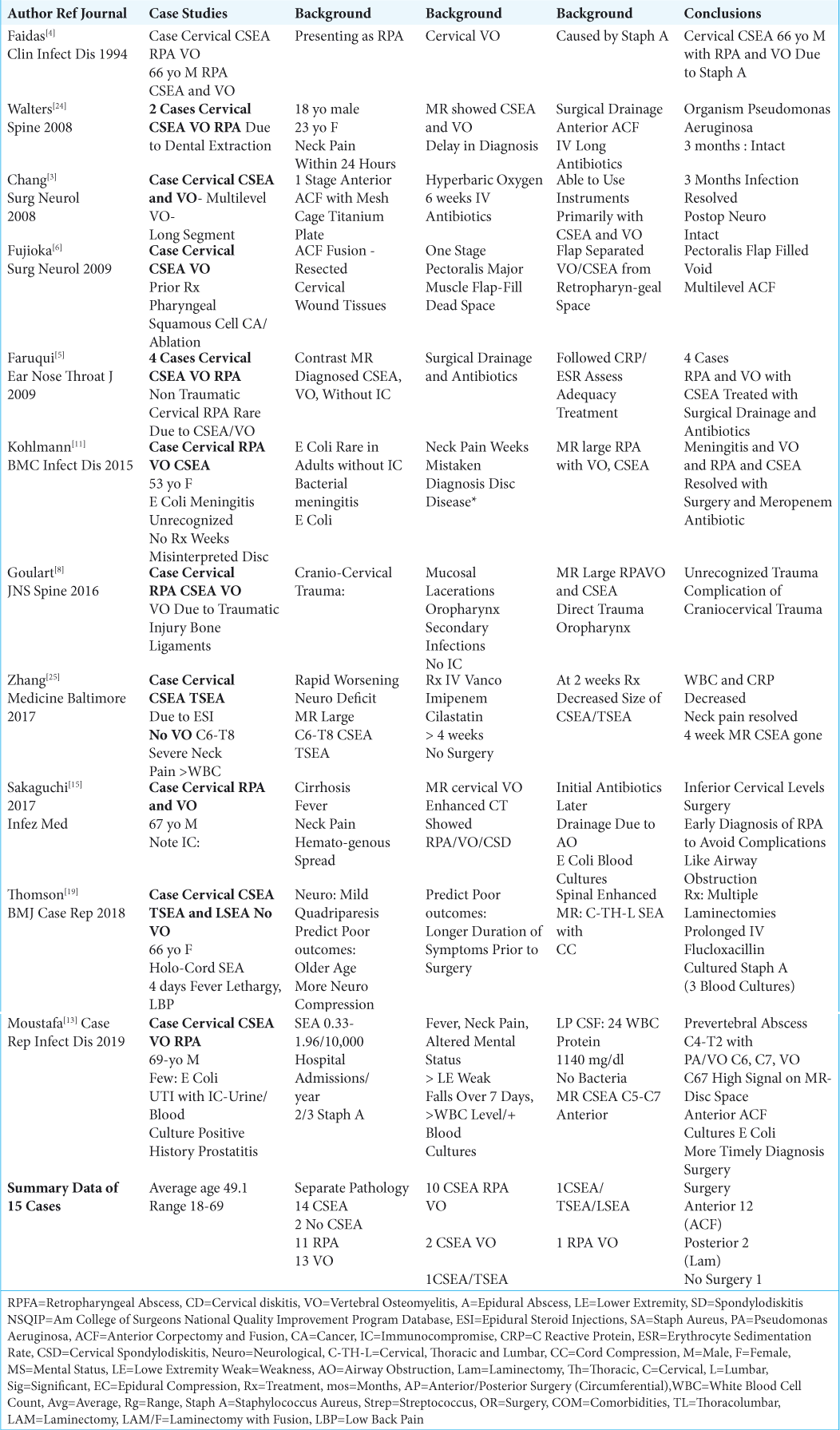
11 CASE STUDIES INVOLVING 15 PATIENTS WITH CERVICAL EPIDURAL ABSCESSES (CSEA) AND/OR CERVICAL VERTEBRAL OSTEOMYELITIS (VO) WITH OR WITHOUT CERVICAL RETROPHARYNGEAL ABSCESS (RPA)
In 11 case studies, there were 15 patients who presented with varying frequencies of cervical epidural abscesses (CSEA: 14 patients), and/or vertebral osteomyelitis (VO: 13 patients) with or without retropharyngeal abscesses (RPA 11 patients) [
Sources and Organisms Associated with CSEA and/or Cervical VO with/without RPA
Distinct sources of infection/organisms for cervical CSEA and/or cervical VO, with/without RPA included: 2 dental extractions (Pseudomonas aeruginosa),[
Surgical Options for CSEA, and/or Cervical VO with/ without RPA
Surgical intervention, warranted in 14 ouf of 15 cases, included 12 anterior procedures (i.e. typically multilevel anterior corpectomy with instrumented fusions), and 2 posterior procedures (i.e. including multilevel laminectomies).[
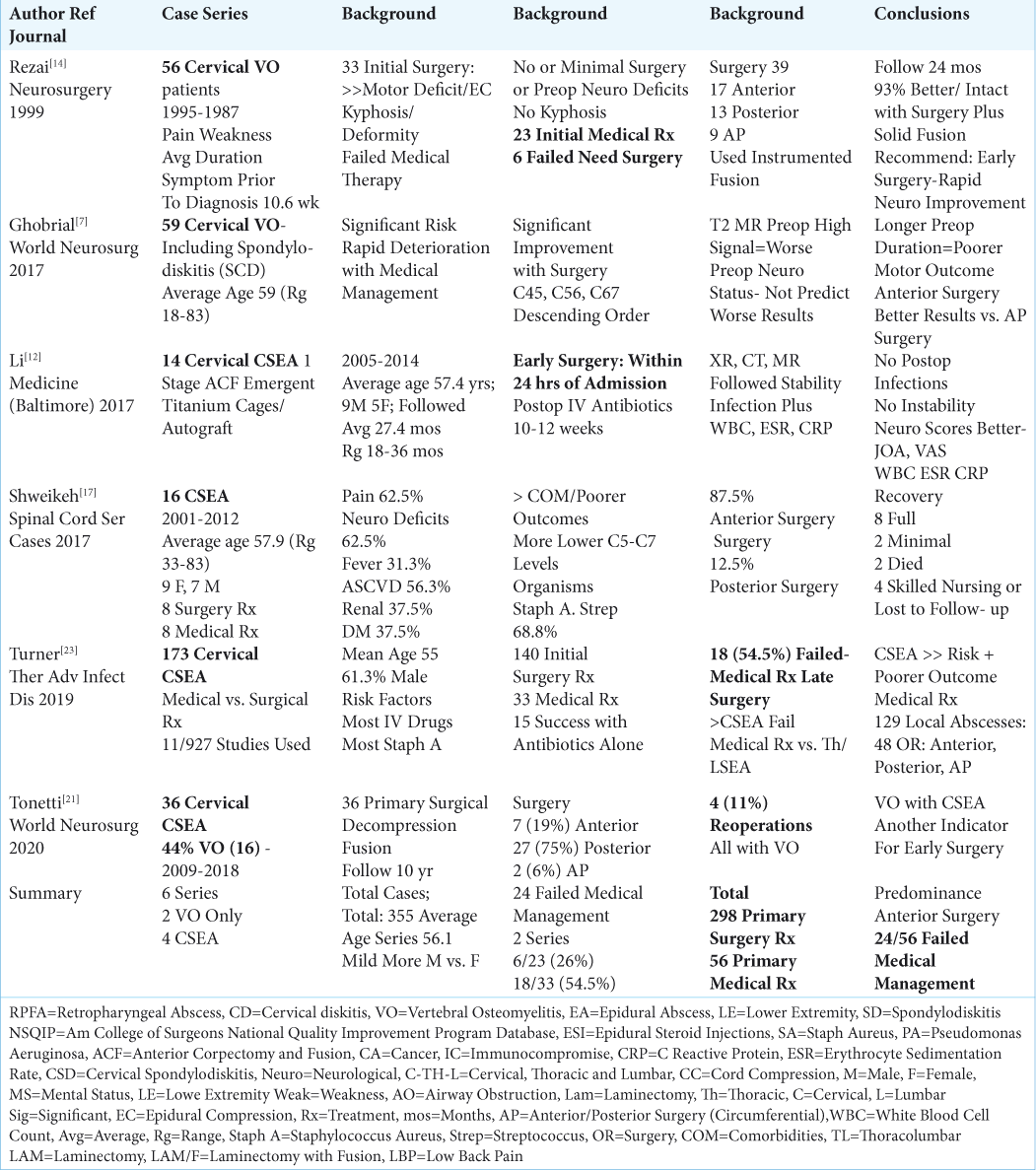
6 LARGER SERIES INVOLVING 355 PATIENTS WITH CERVICAL EPIDURAL ABSCESS (CSEA) AND/OR CERVICAL VERTEBRAL OSTEOMYELITIS (VO) WITH OR WITHOUT CERVICAL RETROPHARYNGEAL ABSCESS (RPA)
There were 6 larger cervical series involving 355 patients included in this analysis; 4 series specifically addressed CSEA (i.e. 1 also including VO), and 2 series involved cervical VO alone [
2 Series Focusing on Cervical Vertebral Osteomyelitis Alone (VO)
Two series focused on the non-surgical and/or surgical management of cervical vertebral osteomyelitis [
4 Series of Cervical Spine Epidural Abscess (CSEA)
From 2017-2020, there were 4 series focusing on CSEA, one of which included VO [
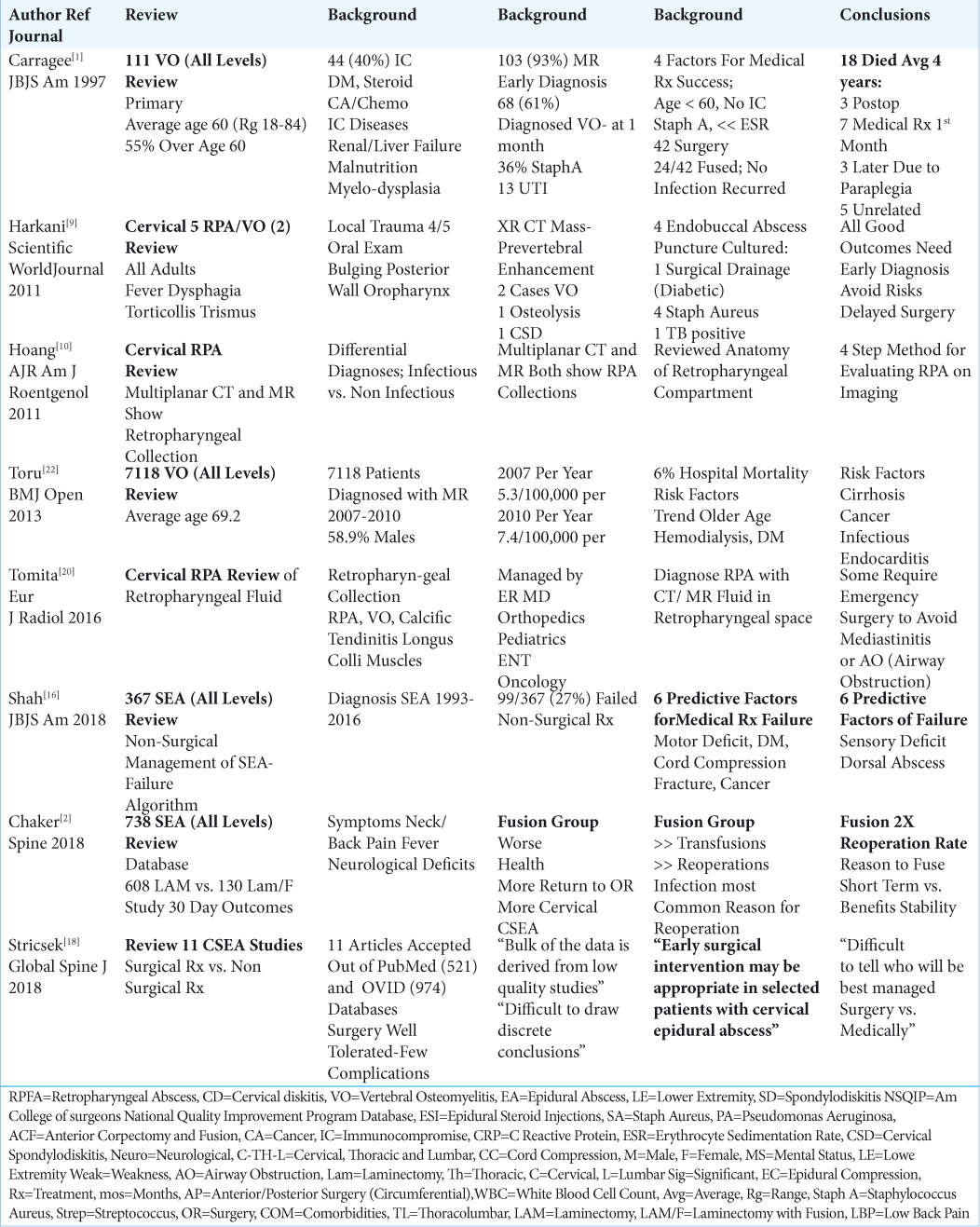
REVIEW OF ARTICLES CONCERNING ALL- LEVEL SEA, VO, AND ADDED REVIEW OF RPA
A review of an additional 8 articles provided a general overview of the diagnosis and treatment of all-level spinal epidural abscesses (SEA), vertebral osteomyelitis (VO), and also discussed more background information regarding RPA [
Frequency and Definition of Cervical Spine Vertebral Osteomyelitis
Vertebral osteomyelitis is seen in hospitals at the rate of approximately 7.4 cases/100,000 patients/per year [
Frequency and Definition of Spinal Epidural Abscesses (SEA)
Spinal epidural abscesses (SEA) occur in approximately 19.6 patients/100,000 per year, and their frequency has tripled over the last 2 decades [
Review of Retropharyngeal Abscesses (RPA)
Cervical retropharyngeal abscesses (RPA) occur in 4.10/100,000 children/year.[
Early diagnosis (e.g. with MR and/or CT) and early surgical treatment were typically critical to avoid respiratory collapse. Most pathogens involved a Staphylococcus aureus species, but others included; Beta-hemolytic Streptococci, anaerobic, and/or Gram-negative organisms. In Harkani et al. (2001) study of 5 patients with RPA, 4 were treated with endobuccal abscess puncture/culture and antibiotics, while 1 required surgical drainage (diabetic); 4 cultured positive for Staph Aureus, while one was positive for Tuberculosis.[
CONCLUSION
Patients presenting with CSEA and/or cervical VO, with or without RPA typically exhibit fever with elevations of WBC, ESR, CRP, and Procalcitonin, have positive blood cultures, and abnormal early diagnostic MR studies (i.e. within 2–4 weeks). With persistent or increasingly elevated laboratory studies, repeatedly positive blood cultures, and progressive pathological findings on MR studies correlating with worsening neurological deficits, early surgery must be considered to maximize recovery, and limit permanent neurological sequelae along with other attendant morbidity, and mortality.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997. 79: 874-80
2. Chaker AN, Bhimani AD, Esfahani DR, Rosinski CL, Geever BW, Patel AS. Epidural Abscess: A Propensity Analysis of Surgical Treatment Strategies. Spine. 2018. 43: E1479-E1485
3. Chang WC, Tsou HK, Kao TH, Yang MY, Shen CC. Successful treatment of extended epidural abscess and long segment osteomyelitis: a case report and review of the literature. Surg Neurol. 2008. 69: 117-20
4. Faidas A, Ferguson JV Jr, Nelson JE, Baddour LM. Cervical vertebral osteomyelitis presenting as a retropharyngeal abscess. Clin Infect Dis. 1994. 18: 992-4
5. Faruqui S, Palacios E, Friedlander P, Melgar M, Alvernia J, Parry PV. Nontraumatic retropharyngeal abscess complicated by cervical osteomyelitis and epidural abscess in post-Katrina New Orleans: four cases. Ear Nose Throat J. 2009. 88: E14-
6. Fujioka M, Oka K, Kitamura R, Yakabe A. Cervical osteomyelitis and epidural abscess treated with a pectoralis major muscle flap. Surg Neurol. 2009. 72: 761-4
7. Ghobrial GM, Franco D, Theofanis T, Margiotta PJ, Andrews E, Wilson JR. Cervical Spondylodiscitis: Presentation Timing and Surgical Management in 59 Patients. World Neurosurg. 2017. 103: 664-670
8. Goulart CR, Mattei TA, Fiore ME, Thoman WJ, Mendel E. Retropharyngeal abscess with secondary osteomyelitis and epidural abscess: proposed pathophysiological mechanism of an underrecognized complication of unstable craniocervical injuries: case report. J Neurosurg Spine. 2016. 24: 197-205
9. Harakani A, Hassani R, Ziad L, Nouri H, Rochdi Y, Raji A. Retropharyngeal Abscess in Adults: Five Case Reports and Review of the Literature. Scientific WorldJournal. 2011. 11: 1623-1629
10. Hoang JK, Branstetter BF, Eastwood JD, Glastonbury CM. Multiplanar CT and MRI of collections in the retropharyngeal space: is it an abscess?. AJR Am J Roentgenol. 2011. 196: W426-32
11. Kohlmann R, Nefedev A, Kaase M, Gatermann SG. Community-acquired adult Escherichia coli meningitis leading to diagnosis of unrecognized retropharyngeal abscess and cervical spondylodiscitis: a case report. BMC Infect Dis. 2015. 15: 567-
12. Li H, Chen Z, Yong Z, Li X, Huang Y, Wu D. Emergency 1-stage anterior approach for cervical spine infection complicated by epidural abscess. Medicine (Baltimore). 2017. 96: e7301-
13. Moustafa A, Kheireldine R, Khan Z, Alim H, Khan MS, Alsamman MA.editors. Cervical Spinal Osteomyelitis with Epidural Abscess following an Escherichia coli Urinary Tract Infection in an Immunocompetent Host. Case Rep Infect Dis. 2019. 2019: 5286726-
14. Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999. 44: 1018-25
15. Sakaguchi A, Ishimaru N, Ohnishi H, Kawamoto M, Takagi A, Yoshimura S. Escherichia coli in a patient with liver cirrhosis. Infez Med. 2017. 25: 169-173
16. Shah AA, Ogink PT, Nelson SB, Harris MB, Schwab JH. Nonoperative Management of Spinal Epidural Abscess: Development of a Predictive Algorithm for Failure. J Bone Joint Surg Am. 2018. 100: 546-555
17. Shweikeh F, Hussain M, Sangtani A, Issa H, Bashir A, Johnson JP. Cervical spine epidural abscess: a single center analytical comparison to the literature. Spinal Cord Ser Cases. 2017. 3: 17036-
18. Stricsek G, Iorio J, Mosley Y, Prasad S, Heller J, Jallo J. Etiology and Surgical Management of Cervical Spinal Epidural Abscess (SEA): A Systematic Review. Global Spine J. 2018. 8: 59S-67S
19. Thomson C. Spinal cord compression secondary to epidural abscess: the importance of prompt diagnosis and management. BMJ Case Rep. 2018. 2018:
20. Tomita H, Yamashiro T, Ikeda H, Fujikawa A, Kurihara Y, Nakajima Y. Fluid collection in the retropharyngeal space: A wide spectrum of various emergency diseases. Eur J Radiol. 2016. 85: 1247-56
21. Tonetti DA, Eichar B, Ares WJ, Kanter AS, Hamilton DK. Should the Presence of Spondylodiscitis Alter the Surgical Treatment of Patients with Symptomatic Ventral Cervical Epidural Abscesses? An Institutional Analysis. World Neurosurg. 2020. p.
22. Toru Akiyama, Hirotaka Chikuda, Hideo Yasunaga, Hiromasa Horiguchi Kivohide Fushimi, Kazuo Saita. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open. 2013. 3: e002412-
23. Turner A, Zhao L, Gauthier P, Chen S, Roffey DM, Wai EK. Management of cervical spine epidural abscess: a systematic review. Ther Adv Infect Dis. 2019. 6: 2049936119863940-
24. Walters HL, Measley R. Two cases of Pseudomonas aeruginosa epidural abscesses and cervical osteomyelitis after dental extractions. Spine. 2008. 33: E293-6
25. Zhang JH, Wang ZL, Wan L. Cervical epidural analgesia complicated by epidural abscess: A case report and literature review. Medicine (Baltimore). 2017. 96: e7789-