- Department of Neurosurgery, University of Foggia, Foggia, Italy.
- Department of Neurosurgery, University of Bari, Bari, Puglia, Italy.
Correspondence Address:
Francesco Carbone, Department of Neurosurgery, University of Foggia, Foggia, Puglia, Italy.
DOI:10.25259/SNI_538_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio Colamaria1, Maria Blagia2, Matteo Sacco1, Francesco Carbone1. Diffuse vertebral metastases from glioblastoma with vertebroepidural diffusion: A case report and review of the literature. 30-Aug-2021;12:437
How to cite this URL: Antonio Colamaria1, Maria Blagia2, Matteo Sacco1, Francesco Carbone1. Diffuse vertebral metastases from glioblastoma with vertebroepidural diffusion: A case report and review of the literature. 30-Aug-2021;12:437. Available from: https://surgicalneurologyint.com/surgicalint-articles/11072/
Abstract
Background: The occurrence of extraneural metastasis in patients diagnosed with glioblastoma (GBM) is rare with an estimated incidence ranging from 0.4% to 2.0%. Short clinical history is believed to be a possible explanation of the paucity of such cases. Furthermore, to date, only few papers describe cases of vertebral metastases from GBM without evidence of synchronous visceral involvement.
Case Description: The authors report on the case of a 46-year-old woman presenting with a history of surgically treated GBM who developed multiple metastases located in the posterior laminae and vertebral bodies with a single dural metastasis at D6-D8 level 5 years after the initial diagnosis. Total-body computed tomography did not show signs of either intracranial recurrence or visceral involvement. Postoperative pathological examination confirmed the diagnosis of the World Health Organization-2016 Grade IV GBM metastases.
Conclusion: From a clinical point of view, the awareness of the possibility of spinal and vertebral metastasis from intracranial GBM is crucial. The present case demonstrates that distant dissemination from the primary tumor is possible despite the absence of intracranial recurrence.
Keywords: Case report, Dural metastasis, Glioblastoma metastasis, Neurosurgery, Vertebral glioblastoma
INTRODUCTION
Glioblastoma (GBM), or 2016 World Health Organization (WHO) diffuse astrocytic and oligodendroglial tumor,[
Until recently, extracranial GBM dissemination was not believed to occur because of the presence of CNS safeguard mechanisms as the blood–brain barrier and the overall short median survival of these patients. However, advancements in early detection and therapy protocols allowed the exponential growth of such condition which is now reported to occur between 0.4% and 2.0% of intracranial GBM cases.[
Hereby, the authors present a case of multiple vertebral bone metastases with further localization in the subdural space in a patient who received the diagnosis of GBM 5 years before.
CASE REPORT
A 46-year-old woman presented in 2016 with a brief history of worsening headaches without any significant medical history. Physical examination was unremarkable and did not indicate any evidence of neurological deficits. Magnetic resonance of the brain demonstrated a right frontoparietal lesion surrounded by vasogenic edema showing avid contrast enhancement. In the suspect of a GBM, a right parietal craniectomy followed by gross-total resection of the tumor was performed and the patient recovered without neurological deficits. Histologic examination confirmed the diagnosis of primary GBM, isocitrate dehydrogenase wild type; World Health Organization 2016 Grade IV, O[
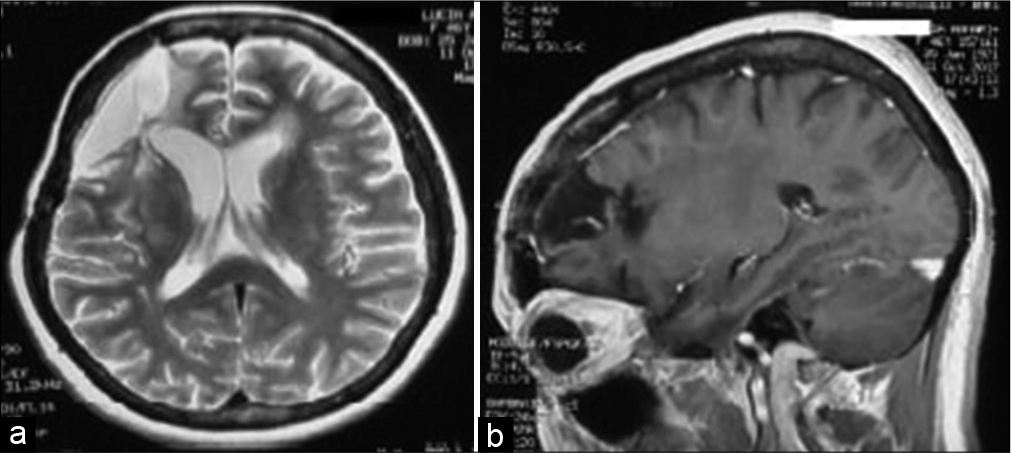
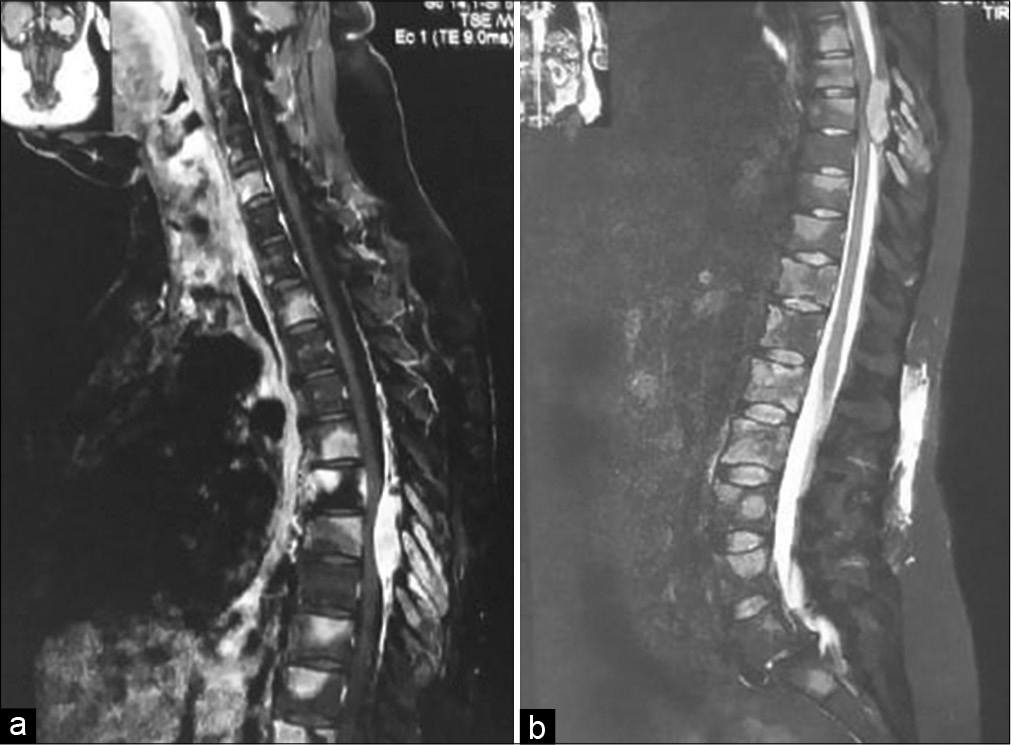
However, 5 years after the initial diagnosis, the patient experienced low back pain irradiating to the legs which was unresponsive to symptomatic pharmacotherapy. Subsequently, she developed numbness and loss of sensitivity in the lower extremities and, in January 2021, was readmitted to our department. Neurological examination revealed a right leg hyposthenia (MRC 3/5) with absent deep tendon reflexes (DTR) and bilateral hypoesthesia at the D5-D6 level. Three days after admission, a sudden worsening of the neurological condition with onset of paraplegia (ASIA A) was noticed. Therefore, an emergency magnetic resonance imaging (MRI) scan of the brain was performed which revealed no evidence of primary site GBM recurrence [
In the hypothesis of epidural metastasis from GBM, the patient was treated with D6-D8 laminectomy followed by partial tumor resection. On intraoperative inspection, the neoplastic tissue presented as ovoidal, white colored, capsulated, and roughly 0.5–1.3 cm in diameter. Microscopic examination demonstrated glial components with marked desmoplasia, deponing for a WHO-2016 Grade IV GBM.[
Postoperatively, sensory and motor deficits persisted; the patient exhibited slight improvement in muscle strength (MRC 4/5) in the lower extremities and reduced but present DTRs. Neurological examination revealed a persistent hypoesthesia of the D5-D6 dermatome. Total-body computed tomography (CT) showed no further localizations of the tumor, therefore, the patient was transferred to a physical rehabilitation center and evaluated for adjuvant radiotherapy. After 6 months, the patient was able to walk although the gait was insecure and slow; she had regained sensation in the lower thoracic dermatomes and brisk reflexes were evoked.
DISCUSSION
Extraneural metastases from GBM represent a rare entity occurring late in the course of the disease, after a median of 2 years and in only 0.2–4% of all patients diagnosed with this highly malignant tumor.[
Notwithstanding recent advancements, pathophysiological pathways of extraneural seeding of tumor cells need to be clarified. Detection of high levels of proteases within neoplastic tissue including urokinase-type plasminogen activator along with lower levels of their inhibitors is believed to be a possible explanation of GBM extradural diffusion.[
Typically, following spinal dissemination, the neurological condition rapidly deteriorates, and management is primarily palliative with most patients undergoing decompressive laminectomy when spinal cord compression is observed.[
In the present report, a case of multiple and diffuse extraneural vertebral metastases from intracranial GBM is described. Although it cannot be excluded, this was a primary spinal localization of the tumor, the author speculates of a possible distant site dissemination in the absence of intracranial recurrence, as was previously described by other groups.[
CONCLUSION
A rare case of extraneural diffuse vertebral bone metastases in a patient previously treated for intracranial GBM is presented. The exceptional survival time length highlights the importance of suspecting distant dissemination of the tumor when progressive changes in the patient’s general condition occur albeit no evidence of primary site recurrence is confirmed on head MRI. As previously reported, rapid deterioration of the neurological picture and sudden onset of paraplegia due to spinal cord compression was seen in this case, and urgent palliative treatment was performed.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alatakis S, Malham GM, Thien C. Spinal leptomeningeal metastasis from cerebral glioblastoma multiforme presenting with radicular pain: Case report and literature review. Surg Neurol. 2001. 56: 33-7
2. Arita N, Taneda M, Hayakawa T. Leptomeningeal dissemination of malignant gliomas. Incidence diagnosis and outcome. Acta Neurochir (Wien). 1994. 126: 84-92
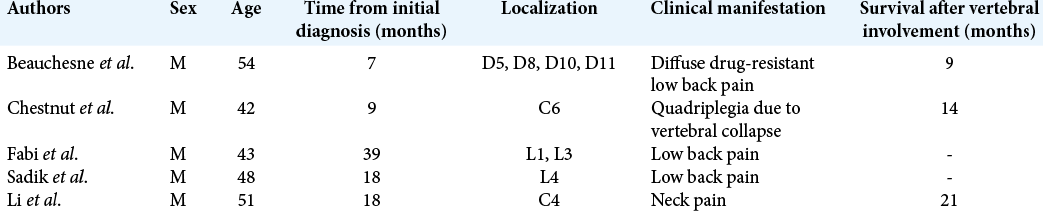
3. Beauchesne P, Soler C, Mosnier JF. Diffuse vertebral body metastasis from a glioblastoma multiforme: A technetium-99m Sestamibi single-photon emission computerized tomography study. J Neurosurg. 2000. 93: 887-90
4. Bindal AK, Hammoud M, Shi WM, Wu SZ, Sawaya R, Rao JS. Prognostic significance of proteolytic enzymes in human brain tumors. J Neurooncol. 1994. 22: 101-10
5. Chesnut RM, Abitbol JJ, Chamberlain M, Marshall LF. Vertebral collapse with quadraparesis due to metastatic glioblastoma multiforme: Case report and review of the literature. J Neurooncol. 1993. 16: 135-40
6. Cunha ML, Maldaun MV. Metastasis from glioblastoma multiforme: A meta-analysis. Rev Assoc Med Bras 1992. 2019. 65: 424-33
7. Dardis C, Milton K, Ashby L, Shapiro W. Leptomeningeal metastases in high-grade adult glioma: Development, diagnosis, management, and outcomes in a series of 34 patients. Front Neurol. 2014. 5: 220
8. Fabi A, Vidiri A, Carapella C, Pace A, Occhipinti E, Caroli F. Bone metastasis from glioblastoma multiforme without central nervous system relapse: A case report. Anticancer Res. 2004. 24: 2563-5
9. Gilard V, Tebani A, Dabaj I, Laquerrière A, Fontanilles M, Derrey S. Diagnosis and management of glioblastoma: A comprehensive perspective. J Pers Med. 2021. 11: 258
10. Goodwin CR, Liang L, Abu-Bonsrah N, Hdeib A, Elder BD, Kosztowski T. Extraneural glioblastoma multiforme vertebral metastasis. World Neurosurg. 2016. 89: 578-82.e3
11. Hansen S, Rasmussen BK, Laursen RJ, Kosteljanetz M, Schultz H, Nørgård BM. Treatment and survival of glioblastoma patients in Denmark: The Danish neurooncology registry 2009-2014. J Neurooncol. 2018. 139: 479-89
12. Li ZG, Zheng MY, Zhao Q, Liu K, Du JX, Zhang SW. Solitary vertebral metastatic glioblastoma in the absence of primary brain tumor relapse: A case report and literature review. BMC Med Imaging. 2020. 20: 89
13. Longee DC, Friedman HS, Phillips PC, Burger PC, Oakes WJ, Heffez D. Osteoblastic metastases from astrocytomas: A report of two cases. Med Pediatr Oncol. 1991. 19: 318-24
14. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
15. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015. 523: 337-41
16. Myers T, Egelhoff J, Myers M. Glioblastoma multiforme presenting as osteoblastic metastatic disease: Case report and review of the literature. AJNR Am J Neuroradiol. 1990. 11: 802-3
17. Pietschmann S, von Bueren AO, Kerber MJ, Baumert BG, Kortmann RD, Müller K. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/gliosarcoma patients with metastases outside of the central nervous system. PLoS One. 2015. 10: e0121592
18. Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: Past, present, and future. Annu Rev Pathol. 2018. 13: 379-94
19. Sadik AR, Port R, Garfinkel B, Bravo J. Extracranial metastasis of cerebral glioblastoma multiforme: Case report. Neurosurgery. 1984. 15: 549-51
20. Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg. 1969. 31: 50-8
21. Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014. 23: 1985-96
22. Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014. 15: e395-403