- Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
Correspondence Address:
Ha Son Nguyen
Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
DOI:10.4103/2152-7806.176133
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nguyen HS, Doan N, Gelsomino M, Shabani S, Mueller W. Dilemmas surrounding the diagnosis of deep brain stimulation electrode infection without associated wound complications: A series of two cases. Surg Neurol Int 10-Feb-2016;7:
How to cite this URL: Nguyen HS, Doan N, Gelsomino M, Shabani S, Mueller W. Dilemmas surrounding the diagnosis of deep brain stimulation electrode infection without associated wound complications: A series of two cases. Surg Neurol Int 10-Feb-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/dilemmas-surrounding-the-diagnosis-of-deep-brain-stimulation-electrode-infection-without-associated-wound-complications-a-series-of-two-cases/
Abstract
Background:When wounds are benign, diagnosis of deep brain stimulation (DBS) electrode infection and associated intraparenchymal infection can be challenging. Only a couple, such cases exist in literature. Since infections of the central nervous system can be life-threatening, prompt diagnosis is necessary to prevent neurological injury. Employed within the appropriate context, magnetic resonance imaging (MRI) of the brain, as well as laboratory data and clinical presentation, may help guide diagnosis.
Case Descriptions:Case 1 - A 55-year-old male with bilateral DBS electrodes and generators (49 days from last procedure), who presented with confusion and fever. Pertinent positive laboratory was white blood cell 20.5K. MRI of the brain showed edema with enhancement along the right DBS electrode. Wound exploration revealed gross purulence in the subgaleal space. The entire system was removed; cultures from subgaleal space revealed Propionibacterium acnes; cultures from electrode were negative. The patient was sent home on antibiotics. Case 2 - A 68-year-old male with a right DBS electrode (11 days from placement), who presented after an unwitnessed fall, followed by confusion and amnesia. Pertinent laboratory examinations were negative. MRI of the brain showed edema with enhancement along the DBS electrode. Wound exploration revealed no infection. The DBS system was left in place; final cultures were negative; no antibiotics were prescribed. Repeat MRI showed resolving fluid-attenuated inversion recovery (FLAIR) signal and contrast enhancement.
Conclusions:Contrast enhancement, T2 FLAIR, and diffusion weighted imaging are influenced by postoperative changes. Caution is stressed regarding dependence on these features for acute diagnosis of infection and indication for electrode removal. Timing of the imaging after surgery must be considered. Other factors, such as systemic signs and abnormal laboratory data, should be evaluated. Based on these guidelines, retrospectively, the patient in Case 2 should not have been rushed for a wound exploration; close observation with serial imaging and laboratory data may have prevented an unnecessary procedure.
Keywords: Cerebral infection, contrast enhancement, deep brain stimulation, edema, fluid attenuated inversion recovery, magnetic resonance imaging
INTRODUCTION
A dreaded complication of deep brain stimulation (DBS) is hardware infection, which may entail additional hospital expenses, hardware removal, and delay of DBS therapy. Infection rates associated with DBS varies from 0% to 15% in various prior studies.[
CASE REPORTS
Patient 1
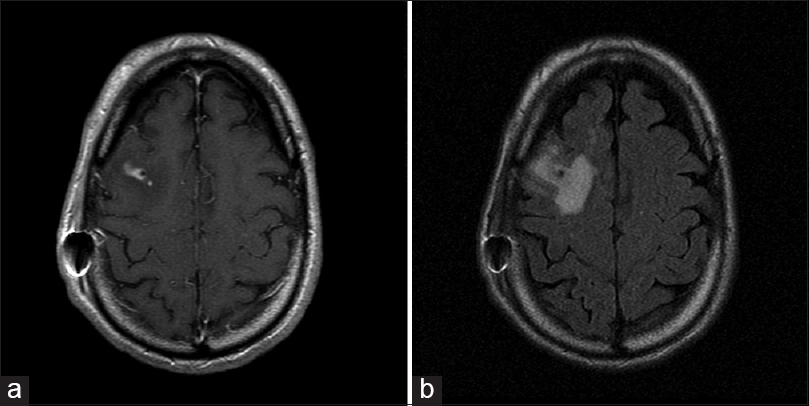
A 55-year-old male, postoperative day 86 (left subthalamic nucleus [STN] electrode), postoperative day 58 (right STN electrode), and postoperative day 49 (bilateral IPG placement), presented to the Emergency Department (ED) with confusion. He was found to have a fever of 102 F in the ED. Pertinent laboratory examinations included lactate 2.0 mmol/L (normal range 0–2.2 mml/L), C-reactive protein (CRP) 1.0 mg/dL (normal range 0–0.5 mg/dL), erythrocyte sedimentation rate (ESR) 17 mm/h (normal range 0–19 mm/h), white blood cell (WBC) 20.5K, UA negative, blood cultures negative, and chest X-ray negative. All incisions were clean, dry, and intact without concerns for infection. CT of the head demonstrated no acute findings. MRI of the brain with contrast demonstrated vasogenic edema along both DBS electrodes, right greater than left, with enhancement tracking around the right DBS electrode [
Patient 2
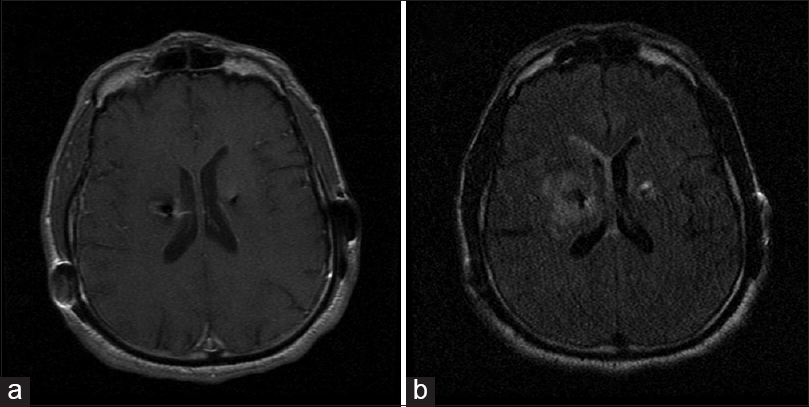
A 68-year-old male, postoperative day 11 (right STN electrode), presented after an unwitnessed fall, followed by confusion and amnesia. He denied fevers, chills, weakness, paresthesias, changes in medications, prior syncopal episodes, or falls. Pertinent laboratory examinations included WBC 9.5, UA negative, ESR 30 mm/h, CRP 1.2 mg/dL, and chest X-ray negative. All incisions were clean, dry, and intact without concerns for infection. CT of the head demonstrated no acute findings. MRI of the brain with contrast demonstrated abnormal edema and enhancement along the DBS electrode [
DISCUSSION
Imaging is a critical component for the diagnosis of an underlying infection, especially when surgical wounds are benign. In particular, MRI has become a diagnostic fixture for CNS infection, especially regarding T1 with contrast, T2 FLAIR, and diffusion-weighted imaging (DWI). However, these protocols may be confusing and misleading, especially during the postoperative period. In particular, contrast enhancement after craniotomy can be variable, appearing as early as 17 h to 5 days postoperatively within nonneoplastic brain parenchyma along surgical margins; moreover, the imaging feature can persist up to 14–29 days postoperatively, though data are limited to 1 month in various studies.[
DWI has become a standard sequence for the differentiation of brain abscess from other ring-enhancing lesions.[
T2/FLAIR signal can be associated with infection or hemorrhage but may also be transient without clinical consequences. Englot et al.[
Inflammatory indices including CRP and ESR have been employed to monitor for infection. However, these indices frequently elevate postoperatively; based on literature regarding orthopedic procedures including spine surgery, these values may take 14 days to 90 days to normalize to preoperative levels.[
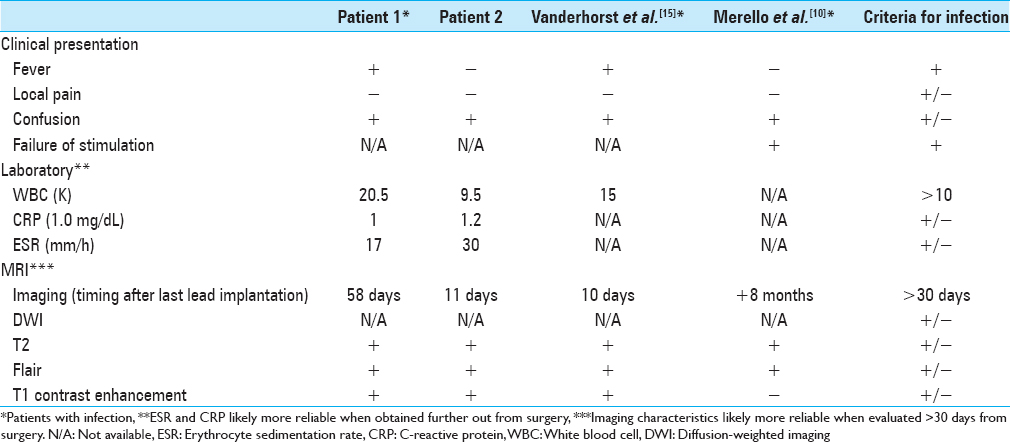
For the rare DBS electrode infections reported in literature without associated wound complications, MRI findings have been inconsistent. Merello et al.[
Considering the influence of postoperative changes to MRI sequences, as well as systemic signs and laboratory data, prompt wound exploration was reasonable for patient 1. He exhibited fevers with an elevated WBC count. Other infectious workup was negative. His last cranial procedure was 58 days prior; consequently, the MRI findings (contrast enhancement and T2 FLAIR) was likely reliable for infection. On the other hand, retrospectively, patient 2 should not have been rushed for a wound exploration. He did not exhibit systemic signs. Moreover, he was only 11 days from electrode placement, where postoperative changes can confound MRI findings and inflammatory indices. Based on the prior literature, contrast enhancement may be benign up to 1 month postoperatively, while T2 FLAIR signal and DWI can remain variable.
CONCLUSIONS
Contrast enhancement, T2 FLAIR, and DWI are influenced by postoperative changes. Caution is stressed regarding dependence on these features for acute diagnosis and indication for electrode removal. Timing of the imaging after surgery must be considered, as postoperative changes can influence all three sequences. Other factors, such as systemic signs (fever/chills) and abnormal laboratory markers (elevated WBC, ESR, and CRP), should be evaluated. Based on these guidelines, retrospectively, patient 2 should not have been rushed for a wound exploration; close observation with serial imaging and laboratory markers may have prevented an unnecessary procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aono H, Ohwada T, Kaneko N, Fuji T, Iwasaki M. The post-operative changes in the level of inflammatory markers after posterior lumbar interbody fusion. J Bone Joint Surg Br. 2007. 89: 1478-81
2. Bhatia S, Zhang K, Oh M, Angle C, Whiting D. Infections and hardware salvage after deep brain stimulation surgery: A single-center study and review of the literature. Stereotact Funct Neurosurg. 2010. 88: 147-55
3. Bjerknes S, Skogseid IM, Sæhle T, Dietrichs E, Toft M. Surgical site infections after deep brain stimulation surgery: Frequency, characteristics and management in a 10-year period. PLoS One. 2014. 9: e105288-
4. Englot DJ, Glastonbury CM, Larson PS. Abnormal T2-weighted MRI signal surrounding leads in a subset of deep brain stimulation patients. Stereotact Funct Neurosurg. 2011. 89: 311-7
5. Farrell CJ, Hoh BL, Pisculli ML, Henson JW, Barker FG, Curry WT. Limitations of diffusion-weighted imaging in the diagnosis of postoperative infections. Neurosurgery. 2008. 62: 577-83
6. Fenoy AJ, Simpson RK. Management of device-related wound complications in deep brain stimulation surgery. J Neurosurg. 2012. 116: 1324-32
7. Forsyth PA, Petrov E, Mahallati H, Cairncross JG, Brasher P, MacRae ME. Prospective study of postoperative magnetic resonance imaging in patients with malignant gliomas. J Clin Oncol. 1997. 15: 2076-81
8. Henegar MM, Moran CJ, Silbergeld DL. Early postoperative magnetic resonance imaging following nonneoplastic cortical resection. J Neurosurg. 1996. 84: 174-9
9. Kong CG, Kim YY, Park JB. Postoperative changes of early-phase inflammatory indices after uncomplicated anterior cervical discectomy and fusion using allograft and demineralised bone matrix. Int Orthop. 2012. 36: 2293-7
10. Merello M, Cammarota A, Leiguarda R, Pikielny R. Delayed intracerebral electrode infection after bilateral STN implantation for Parkinson's disease.Case report. Mov Disord. 2001. 16: 168-70
11. Park KK, Kim TK, Chang CB, Yoon SW, Park KU. Normative temporal values of CRP and ESR in unilateral and staged bilateral TKA. Clin Orthop Relat Res. 2008. 466: 179-88
12. Sato N, Bronen RA, Sze G, Kawamura Y, Coughlin W, Putman CM.editors. Postoperative changes in the brain: MR imaging findings in patients without neoplasms. Radiology. 1997. 204: 839-46
13. Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007. 27: 525-51
14. Smith JS, Cha S, Mayo MC, McDermott MW, Parsa AT, Chang SM. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: Distinguishing tumor recurrence from postresection injury. J Neurosurg. 2005. 103: 428-38
15. Vanderhorst VG, Papavassiliou E, Tarsy D, Shih L. Early brain abscess: A rare complication of deep brain stimulation. Mov Disord. 2009. 24: 1395-7
16. Xu XX, Li B, Yang HF, Du Y, Li Y, Wang WX. Can diffusion-weighted imaging be used to differentiate brain abscess from other ring-enhancing brain lesions? A meta-analysis. Clin Radiol. 2014. 69: 909-15