- Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, New York, India
Correspondence Address:
Mahlon D. Johnson
Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, New York, India
DOI:10.4103/2152-7806.196367
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mahlon D. Johnson. Do mesothelin/MUC16 interactions facilitate adenocarcinoma metastases to intracranial meningiomas?. 21-Dec-2016;7:
How to cite this URL: Mahlon D. Johnson. Do mesothelin/MUC16 interactions facilitate adenocarcinoma metastases to intracranial meningiomas?. 21-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/do-mesothelinmuc16-interactions-facilitate-adenocarcinoma-metastases-to-intracranial-meningiomas/
Abstract
Background:Meningiomas have been shown to express mesothelin, a high affinity binding site for MUC16, a transmembrane protein on adenocarcinoma cells. The mechanisms underlying adenocarcinoma metastases to meningiomas may provide insight into tumor-to-tumor metastases and adenocarcinoma metastases to leptomeningeal cells.
Methods:Two meningiomas containing metastases from adenocarcinomas were identified and evaluated immunohistochemically for the expression and localization of mesothelin and MUC16.
Results:Both meningiomas show extensive mesothelin immunoreactivity, and the adenocarcinomas metastatic to the meningiomas show mesothelin and MUC16 immunoreactivity at the interface with meningioma.
Conclusions:Interactions between MUC16 and/or mesothelin on the cell membrane of adenocarcinoma cells with mesothelin on meningioma cells may facilitate adenocarcinoma metastases to meningiomas and possibly the leptomeninges.
Keywords: Meningiona, mesothelin, metastasis, MUC16, tumor-to-tumor
INTRODUCTION
Adenocarcinomas are one group of carcinomas known to metastasize to the leptomeninges.[
Transmembrane mucins such as MUC1 and MUC16 are thought to facilitate the metastases of many carcinomas, including pulmonary adenocarcinomas.[
Mesothelin is a 40kDa glycosyl-phosphatidylinositol-anchored cell surface protein that has been identified in low levels in mesothelial cells of the pleura, pericardium, and peritoneum.[
Recently, it has been shown that mesothelin binds MUC16, a type I transmembrane protein that belongs to the mucin family of glycoproteins. It is also called CA125.[
MATERIALS AND METHODS
Two meningiomas with intratumoral adenocarcinoma were identified in the University of Rochester archives and consultative material after obtaining Institutional Review Board approval. The first was from a 74-year-old male with a right frontal transitional meningioma. He had a known lung mass. The second patient was a 66-year-old female with a left sphenoid wing meningioma and an adenocarcinoma identified 2 years earlier.
Immunohistochemistry
Each case was analyzed with a monoclonal antibody to human mesothelin.[
RESULTS
Pathology
Patient 1. The sections revealed a transitional, meningioma containing a relatively circumscribed, poorly differentiated adenocarcinoma with clear cell features and necrosis. The metastasis exhibited vimentin, cytokeratin 7, TTF-1, and AE1/AE3, however, no cytokeratin 20 or S-100 immunoreactivity. The adenocarcinoma had clear periodic acid schiff (PAS) and dPAS negative cytoplasm, large pleomorphic nuclei with prominent nucleoli, and focal glandular formation and necrosis. Ki-67 labeling was brisk in the metastasis and approximately 6% in the meningioma. The meningioma had numerous whorls and rare mitoses, but no loss of lobularity, with only modest cellularity and no definite small cell component. The PAS/PAS-D stain revealed no clear cell component.
Patient 2. The meningioma was transitional with atypical features, including hypercellularity, focal loss of lobular pattern, small cell change, and focal necrosis. The meningioma showed extensive epithelial membrane antigen (EMA) but no CAM 5.2, cytokeratin 7, TTF-1, napsyn, or PAS staining. The metastatic adenocarcinoma shows gland formation. The epithelioid cells had prominent nucleoli, high mitotic activity, and necrosis and Kreyberg staining. The carcinoma cells showed EMA, Cam 5.2, cytokeratin 7, napsyn, TTF-1, and Ki-67 labeling of 60%.
Immunohistochemistry
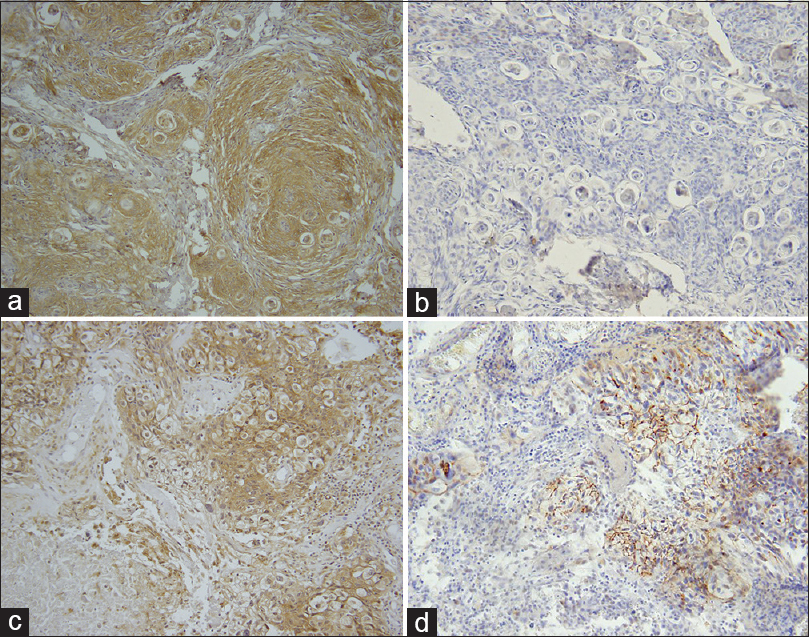
Mesothelin immunoreactivity was detected in both meningiomas and was extensive [Figure
Figure 1
Mesothelin and MUC16 expression in meningiomas with adenocarcinoma metastasis in patient 1. Meningioma with mesothelin immunoreactivity (a) but no MUC16 (b). Metastatic adenocarcinoma to meningioma showing mesothelin (c) and MUC16 (d) Hematoxylin counterstain and diaminobenzidine chromagen (Original magnification, ×400)
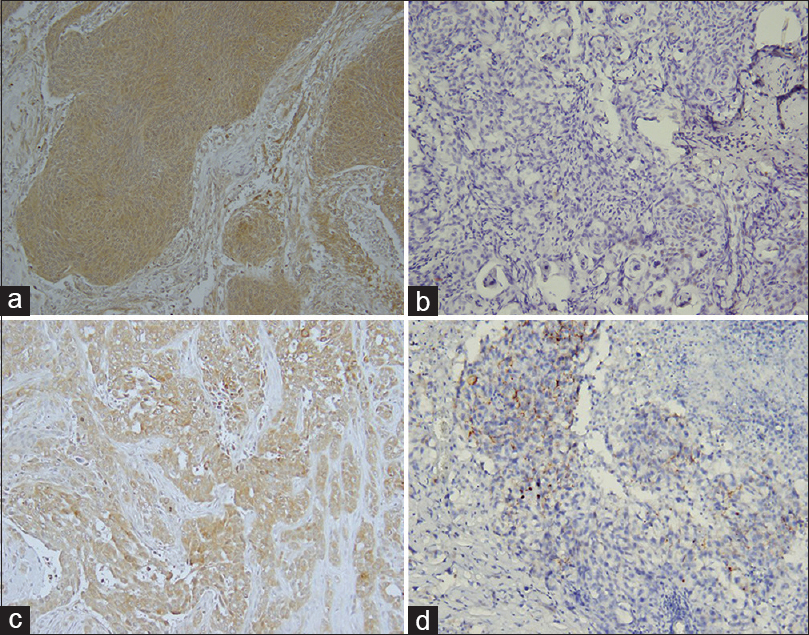
Figure 2
Mesothelin and MUC16 expression in meningiomas with adenocarcinoma metastasis in patient 2. Meningioma with mesothelin immunoreactivity in meningioma (a) and in adenocarcinoma metastatic to meningioma (c). Lack of MUC 16 in meningioma (b) but extensive immunoreactivity in adenocarcinoma (d) Hematoxylin counterstain and diaminobenzidine chromagen (Original magnification, ×400)
Muc16 immunoreactivity was not detected in either meningioma [Figure
DISCUSSION
Previously, we have demonstrated mesothelin expression in the majority of meningioma and 30% of human leptomeninges tested.[
In contrast, meningiomas show widespread mesothelin expression and may have more favorable binding sites for MUC16-expressing carcinomas. Consistent with this is our finding of widespread mesothelin in both meningiomas as well as MUC16 in both carcinomas. Thus, these interactions may represent another mechanism by which tumors metastasize to remote tissues (and tumors).[
Mesothelin may also facilitate metastases by other mechanisms. Recent studies have shown that mesothelin induces expression of metaloproteinase MMP9, which has been implicated in tumor invasion.[
Targeting mesothelin with an anti-mesothlin immunotoxin SS1P binding has been shown to cause regression of mesothelin-expressing tumors in athymic mice. It also has exhibited tumoricidal effects on mesotheliomas and ovarian carcinomas.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agalioti T, Giannou AD, Stathopoulos . Pleural involvement in lung cancer. J Thor Dis. 2015. 7: 1021-30
2. Bhargava P, McGrail KM, Manz HJ, Baidas S. Lung carcinoma presenting as metastasis to intracranial meningioma: Xase report and review of the literature. Am J Clon Oncol. 1999. 22: 199-202
3. Brown GT, Muttay GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumor invasion and metastasis. J Pathol. 2015. 237: 273-81
4. Carr K, Hwe L, Weaver K, Highfield Nickols H. Renal cell carcinoma metastatic to meningioma: Tumor-tumor metastasis. Clin Neuropathol. 2014. 33: 152-6
5. Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas and ovarian cancers. Proc Natl Acad Sci U S A. 1996. 93: 136-40
6. Glass R, Hukku S, Gershenhorn B, Alzate J, Tarr B. Metastasis of adenosquamous carcinoma to meningioma: Case report with literature review. Int J Clin Exp Pathol. 2013. 6: 2625-30
7. Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault CM, Ho M. Mesothelin- MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006. 5: 50-65
8. Hamperl M, Goehre F, Schwan S, Jahromi BR, Ludka CM, Mendel T. Tumor-to-tumor metastasis - Bronchial carcinoma in meningioma. Clin Neuropathol. 2015. 34: 302-6
9. Hassan R, Bera T, Pastan I. (2004) Mesothelin: A new target for immunotherapy. Clin Cancer Res. 2004. 10: 3937-42
10. Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004. 4: 45-60
11. How E, Lee R, Kasem K, Withers T. Surprise within a meningioma: Case report of a signet ring cell carcinoma in a meningioma. ANZ J Surg. 2015. 10: 1111-
12. Johnson MD, O’Connell M, Vito F. Mesothelin in leptomeninges and meningiomas. J Histochem Cytochem. 2008. 56: 579-85
13. Johnson M MD, Vito F, Xu H. MUC16 Expression and Risk of Adenocarcinoma Metastases to Peritoneum, Pleura, Leptomeninges and Brain. J Appl Immunohistochem Mol Morphol. 2010. 18: 250-3
14. Kelly RJ, Sharon E, Pastan IR, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012. 11: 517-25
15. Kim KH, Hong EK, Lee SH, Yoo H. Non small cell carcinoma metastasis to meningioma. J Korean Neurosurg Soc. 2013. 53: 43-5
16. Kojima T, Oh-eda M, Hattori K, Taniguchi Y, Tamura M, Ochi N. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J Biol Chem. 1995. 270: 21984-90
17. Okada E, Nakamura M, Koshida Y, Mukai K, Toyama Y, Matsumoto M. Breast carcinoma metastasis to meningioma in the thoracic spine: A case report and review of the literature. J Spinal Cord Med. 2015. 38: 231-5
18. Onda M, Willingham M, Nagata S, Bera TK, Beers R, Ho M, Hassaet al. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting, western blotting and ELISA. Clin Cancer Res. 2005. 11: 5840-6
19. Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003. 27: 1418-28
20. Ravnik J, Ravnik M, Bunc G, Glumbic I, Tobi-Veres E, Velnar T. Metastasis of an occult pulmonary carcinoma into meningima: A case report. World J Surg Oncol. 2015. 13: 292-
21. Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M. Binding of ovarian cancer cell antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004. 279: 9190-8
22. Sayegh ET, Henderson GA, Burch EA, Reis GF, Cha S, Oh T. Intrameningioma metastasis of breast carcinoma. Rare Tumors. 2014. 6: 5313-
23. Servais EL, Colovos C, Rodriquez LA, Bograd AJ, Nitadori J, Sima C. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res. 2012. 18: 2478-89
24. Smalley RV, Malmud LS, Ritchie WG. Preoperative scanning: Evaluation for metastatic disease in carcinoma of the breast, lung, colon and prostate. Semin Oncol. 1980. 7: 358-69
25. Svokos KA, Salhia B, Toms SA. Molecular biology of brain metastasis. Int J Mol Sci. 2014. 15: 9519-30
26. Talukdar A, Khanra D, Mukhopadhay S, Bose D. Tumor-to-tumor metastasis: Adenocarcinoma of lung metastatic to meningioma. J Post Grad Med. 2014. 60: 403-5
27. Weible UH, Birzele F, Kollmorgen G, Ruger R. Dissection of the process of brain metsatasis reveals targets and mechanisms for molecular-based intervention. Cancer Genomics Proteomics. 2016. 13: 245-58
28. Wesseling P, von Deimling A, Aldape KD, Preusser M, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editors. Metastatic tumours of the CMS. Tumours of the Nervous System. Geneva, Switzerland: WHO Press; 2016. p. 338-41
29. Yamaguchi N, Hatori K, Oh-eda M, Kojima T, Imai N, Ochi N. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y5. J Biol Chem. 1994. 269: 805-8
30. Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 2008. 8: 3329-41