- Department of Neurosurgery, The Walton Centre, Liverpool, United Kingdom.
Correspondence Address:
Oluwaseyi Adebola, Department of Neurosurgery, The Walton Centre, Liverpool, United Kingdom.
DOI:10.25259/SNI_50_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Oluwaseyi Adebola. Do we need a neurosurgical frailty index?. 19-Apr-2024;15:134
How to cite this URL: Oluwaseyi Adebola. Do we need a neurosurgical frailty index?. 19-Apr-2024;15:134. Available from: https://surgicalneurologyint.com/surgicalint-articles/12865/
Abstract
Background: An increasing number of elderly patients now require neurosurgical intervention, and it is sometimes unclear if the benefits of surgery outweigh the risks, especially considering the confounding factor of numerous comorbidities and often poor functional states. Historically, many patients were denied surgery on the basis of age alone. This paper examines the current selection criteria being used to determine which patients get offered neurosurgical management and attempts to show if these patients have a good outcome. Particular focus is given to the increasing insight into the need to develop a neurosurgical frailty index.
Methods: Using a prospective cohort study, this study observed 324 consecutive patients (n) over a 3-month period who were ≥65 years of age at the time of referral or admission to the neurosurgical department of the Royal Hallamshire Hospital. It highlights the selection model used to determine if surgical intervention was in the patient’s best interest and explores the reasons why some patients did not need to have surgery or were considered unsuitable for surgery. Strengths and weaknesses of different frailty indices and indicators of functional status currently in use are discussed, and how they differ between the patients who had surgery and those who did not.
Results: Sixty-one (18.83%) of n were operated on in the timeframe studied. Compared to patients not operated, they were younger, less frail, and more functionally independent. The 30-day mortality of patients who had surgery was 3.28%, and despite the stringent definition of poor outcomes, 65.57% of patients had good postoperative results overall, suggesting that the present selection model for surgery produces good outcomes. The independent variables that showed the greatest correlation with outcome were emergency surgery, the American Society of Anesthesiology grade, the Glasgow Coma Scale, and modified frailty index-5.
Conclusion: It would be ideal to carry out future studies of similar designs with a much larger sample size with the goal of improving existing selection criteria and possibly developing a neurosurgical frailty index.
Keywords: Neurosurgical frailty index, Frailty, Neurosurgery in elderly, Neurosurgery in frail patients
INTRODUCTION
Background
Current data suggest that about 11% of the world’s population is at least 60 years of age with this figure expected to rise to 22% by 2050.[
Frailty
Frailty has been progressively shown to be a very important index for predicting postoperative complications and thus weighing up the risk of surgery against the likely benefit. Clegg et al.[
What is the best frailty index and determinant of functional status?
Some of the commoner indices currently in use have undergone different degrees of validation and modification, but it appears that it is still quite elusive to get robust indices which are easy to administer or derive in the clinical setting, capture the imperative information, and yet have high predictive values.
The MFI-5 used in this paper was derived from MFI-11 which has 11 factors made of 16 variables. One criticism of the MFI-5 noted while carrying out this research work is that it appears to categorize certain patients erroneously. A patient who is frail from any other combination of morbidities apart from congestive heart failure, hypertension, diabetes, or chronic obstructive pulmonary disease and is not currently functionally dependent will be assigned a score of 0 even though such a patient may have glioblastoma multiforme with just a few months to live and have other comorbidities not recognized by the MFI-5. The Clinical frailty scale [
The Glasgow Coma scale (GCS)[
The Eastern Cooperative Oncology Group/World health organization performance status[
The modified Rankin scale (MRS) [
It is, therefore, apparent that more work needs to be done in the creation of a frailty index, perhaps primarily created for neurosurgical patients, that will be reproducible, easy, and convenient to determine and have a well-validated predictive value of neurosurgical outcomes of interest.
The status quo
At present, the majority of works in the literature that have studied the impact the age of patients has on their outcomes after neurosurgical intervention are retrospective and single-center studies. These include the works of Chen et al.[
An ideal study would be prospective, multicenter, involve a large study number of patients, and take into cognizance pre-and post-operative functional status, presence of neurologic deficits, and the impact of frailty. It is nonetheless understandable that such a project would require significant resources and commitment.
Bligh et al[
Plan of investigation
Research objective
This project aims to evaluate if elderly patients undergoing neurosurgery have a good outcome based on current selection criteria. The objective is to explore the criteria currently employed in determining which elderly patients will be operated on and the impact pre-operative functional status and frailty, in particular, have on determining outcomes following neurosurgical intervention. The hypothesis is that the patient’s outcome postoperatively will be better predicted by the patient’s pre-morbid functional status and/or presence of frailty rather than the patient’s age alone.
MATERIALS AND METHODS
Consecutive patients referred or admitted to the neurosurgery department of Royal Hallamshire Hospital were recruited to the study over three months from April to June 2019. Elderly patients were defined as patients who were 65 years and above at the time of referral or admission for elective and emergency procedures.[
Patient demographics, medical history, functional status, indication for surgery or reason for referral, surgery performed, and 30-day outcomes were collected prospectively in a patient registry. Functional status was assessed using the Karnofsky performance scale[
Statistics
Outcomes were assessed using postoperative functional status as determined by the mRS, and also the 30-day morbidity and mortality.[
For this study, P < 0.05 has been employed to show statistical significance. Statistical analysis was performed using RStudio version 1.2.1335 (RStudio, Boston, Massachusetts, USA) and GraphPad Prism 8 (2365 Northside Dr., Suite 560, San Diego, California).
RESULTS
A total number of 324 patients above 65 years of age were either referred or admitted to the neurosurgery department of Sheffield Teaching Hospital during the period under review. This includes patients who were admitted for elective procedures and patients with non-urgent, urgent, or emergency referrals for neurosurgical input.
The youngest patient was 65 years, and the oldest patient was 97 years, while the mean age of the study was 78 years. Of this, 49.07% were male and 50.93% were female. Eighty-one of these patients had suffered a cerebrovascular accident; 51 patients had spinal pathologies such as lumbar canal stenosis or suspected cauda equina syndromes; 53 patients had brain or spinal tumors – This includes many incidental meningiomas and malignant gliomas. Thirty-four patients had chronic subdural hematomas, while 25 patients were referred with acute subdural hematomas.
Only 61 (18.83%) out of the 324 patients who met the selection criteria for this study had an operation. About 18.83% of the 324 patients in total (n) died within one month of referral or admission, while the 30-day mortality rate of the operated patients was 3.28%.
Operated patients
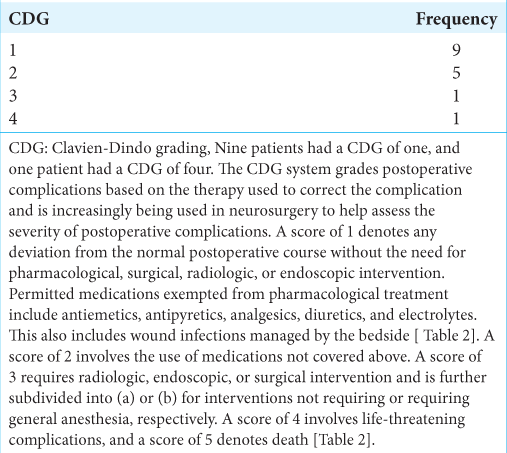
Of all the patients who had surgery, 27 were elective admissions, 14 were emergency referrals/admissions, 19 were urgent referrals, and one was a non-urgent referral. Ten of these patients (16.39%) had been previously admitted to the neurosurgery department in the preceding 12 months. Sixteen (26.23%) of the patients operated had varying degrees of postoperative complications. The postoperative mortality rate for patients who underwent surgery was 3.28%.
Operated versus not-operated comparisons
Patients who were operated on (Group A) were relatively younger, with a mean age of 75.5 (P = 0.00243). They had a lower mean WHO performance status of 2.42 compared to 3.14 in Group B, who did not have surgery (P = 0). They had a lower mean American Society of Anesthesiology (ASA) of 2.80 compared to 3.54 in Group B (P < 0.05). The mean pre-morbid karnofsky performance status (KPS) [
Good outcome versus poor outcome
The second objective of this paper is to find out if patients have a good outcome based on the current selection criteria used. Poor outcomes include characteristics already defined, and it should be noted that patients with a CDG of 1–3 have been included in this list.
DISCUSSION
Historical background
Munro et al[
Brandes et al. (Brandes, 2003)[
Present work
Only 61 (18.83%) out of the 324 patients included in this work actually had surgery during the timeframe examined. This is understandably so considering that a sizeable number of patients who did not need neurosurgery. For example, 81 of the patients referred to the department (25% of n) had suffered a cerebrovascular accident, and as mirrored in many previous publications such as Rabinstein et al. (Rabinstein, 2002),[
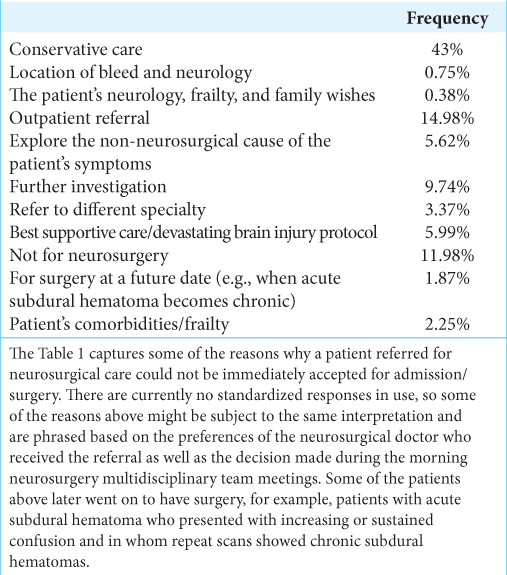
Sixteen (5.99%) of the patients who did not merit neurosurgical intervention in this current work were recommended for best supportive care or commenced on devastating brain injury protocol. One hundred and fifteen patients were recommended for conservative care. Many of these patients had pathologies for which urgent neurosurgical care was not deemed necessary based on universal brain trauma guidelines.
Lewis et al.[
Five patients in this study were refused initial neurosurgical intervention with a plan for possible future surgery. This was the case in patients with acute subdural hematomas (aSDH). Many studies have shown that surgery for acute subdural hematomas are usually associated with high mortality rates. One such work is by Benedetto et al.,[
Comparison of operated versus not-operated patients
This paper has categorized every patient who did not have surgery in the period studied as “Not-Operated.” This group includes patients who were deliberately managed conservatively, patients who were deemed inappropriate referrals to neurosurgery, patients who needed further work-up before a decision on surgery could be made, and patients who might have benefited from surgery but were considered too frail.
Patients who were managed surgically were younger than patients who were not with mean ages of 75.51 and 78.91, respectively (P = 0.002). They also had better indices of frailty including ASA, WHO performance status[
The mortality rate in the patients who were not operated on was significantly higher than the rate in patients who were managed surgically, with values of 23.04% and 3.28%, respectively. It is understandable why this is the case as while the operated group included many elective procedures, such as spinal procedures for lumbar decompression, which have a relatively low mortality rate[
Good versus poor outcome
The second part of the objective of this paper was to try to determine if patients who were selected for surgery based on present selection criteria went on to have good outcomes. We were also interested in knowing how patients who had good outcomes differed from patients who had bad or poor outcomes. Forty of the 61 patients operated on (65.57%) had good outcomes, while 21 patients (34.43%) had poor outcomes. The 30-day mortality rate of patients who had surgery is considered reasonably low at 3.28%. Bligh et al.[
We included CDG of 1 and 2 in poor outcomes due to the stringent criteria used in this study. This is similar to the criteria used by Maldaner et al. [
With a P-value of 0.78, it is assumed that there was no statistically significant difference between the ages of patients who had good outcomes (Group A) compared to those who did not (Group B). Surprisingly also, comparison in GCS and majority of the frailty indices between Group A and B patients did not reveal any statistically substantial difference. The GCS was marginally higher in Group A with a value of 14.85 compared to 13.91 in Group B; however, the P-value was 0.76 so we cannot reject the null hypothesis. Focal deficits were equally present in both groups of patients. This is contrary to expectations, where it would have been anticipated that patients with a greater degree of preoperative focal deficits would have a greater burden of postoperative complications. In the same vein, the MFI-5 was observed to be slightly lesser in patients with poor outcomes (0.43 as against 1.05 in Group A) with a P-value of 0.0056. This would suggest a lesser burden of comorbidities in the patients who had poor outcomes and would be against the run of play. It would be interesting to observe what the findings will show if a much larger sample size is used.
ASA in Group B was marginally higher, with a value of 3.33 as against 2.53 in Group A, but with a P-value of 0.75. P-values of variations between the KPS and Clinical Frailty Index of Group A and Group B patients were 0.77 and 0.09, respectively, which would suggest that the observed numerical values between the two groups are statistically negligible.
Predictors of poor outcomes
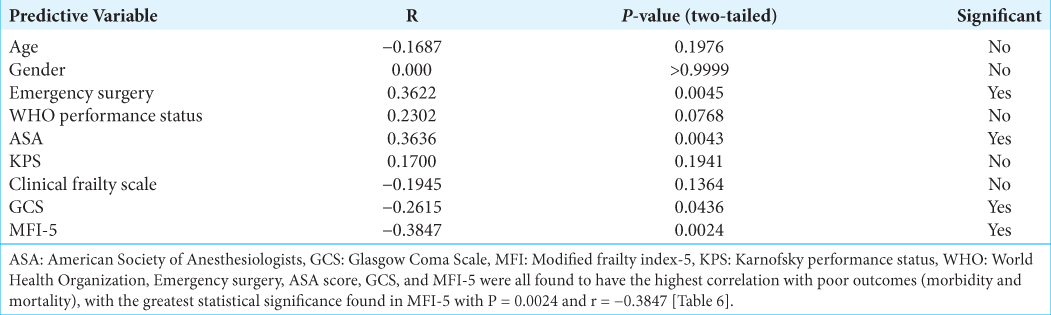
Finally, an attempt was made to show what patient factors were the greatest predictors of poor outcomes. Pearson’s correlation revealed that emergency surgery, ASA score, GCS, and MFI-5 all showed a statistically significant correlation with poor outcomes. The greatest significance was observed in MFI-5 with P = 0.0024 and r = −0.3847. These findings support the hypothesis that a patient’s premorbid functional status and degree of frailty rather than age in isolation may be used to deduce the possibility of poor postoperative outcomes reasonably.
Ideally, logistic regression analysis may best serve to find out the predictors of mortality as employed by Bligh et al.[
n = 10 k/p
Limitations
The major challenge in writing this paper was its relatively small sample size. This was due to a combination of the prospective nature of the research work and the short time frame under which it had to be done. This probably impacted the ability to show statistical significance and, as demonstrated above, the derivation of logistic regression between postoperative outcomes and the multiple predictive variables of interest. Furthermore, the follow-up postoperatively for many of this subsection of patients was set at 6–8 weeks after surgery. As discussed earlier, for these patients and those lost to follow-up for any reason, functional outcomes were judged based on the patient’s performance at the time of discharge. It is, however, reasonable to expect that with physiotherapy and pain resolution, many of these patients will have developed greater functional independence 2–3 months after surgery, further increasing the percentage of good outcomes observed.
Finally, the majority of patients included in this study were referred from trusts other than Sheffield Teaching Hospitals National Health Service Foundation Trust and not admitted to Royal Hallamshire Hospital. Some of them did not have their medical records linked to the Personal Demographics Service leading to gaps in data on past medical records, place of residence, and adequate records of 30-day functional status.
CONCLUSION
The existing selection criteria for neurosurgery in the elderly are associated with a good overall outcome. Frailty and preoperative functional status correlate more with good outcomes rather than age as a stand-alone consideration. Therefore, it is increasingly important that this is factored in when making a clinical decision on who is expected to benefit reasonably from neurosurgical procedures.
Neurosurgical frailty index
There appears to be a need to develop a neurosurgical frailty index whose design will better incorporate the characteristics necessary in the context of an elderly patient in need of neurosurgical care. Current models in use are mostly borrowed from sister specialties and might not adequately identify the cognitive and functional subtleties needed to categorize postoperative neurosurgical outcomes. It will also be ideal to carry out the research work just completed prospectively over a longer duration and across multiple centers.
Hopefully, this work will set the pace for future researchers to carry out more studies in comparing which frailty indices best predict good outcomes in elderly patients who have neurosurgery. The results of this may go on to form the basis for a neurosurgical frailty index and help improve the selection criteria currently in use.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Benedetto NG, Gambacciani C, Montemurro N, Morganti R, Perrini P. Surgical management of acute subdural haematomas in elderly: Report of a single center experience. Br J Neurosurg. 2017. 31: 244-8
2. Bligh ER, Sinha P, Smith D, Al-Tamimi YZ. Thirty-day mortality and survival in elderly patients undergoing neurosurgery. World Neurosurg. 2020. 133: e646-52
3. Brandes AA, Vastola F, Basso U, Berti F, Pinna G, Rotilio A. A prospective study on glioblastoma in the elderly. Cancer. 2003. 97: 657-62
4. Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke. 2017. 48: 2007-12
5. Cesari M. Why and how do we measure frailty?. Intern Emerg Med. 2019. 14: 5-6
6. Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky performance scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol. 2015. 121: 359-64
7. Chen ZY, Zheng CH, Li T, Su XY, Lu GH, Zhang CY. Intracranial meningioma surgery in the elderly (over 65 years): Prognostic factors and outcome. Acta Neurochir (Wien). 2015. 157: 1549-57 discussion 1557
8. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013. 381: 752-62
9. Cloney M, D’Amico R, Lebovic J, Nazarian M, Zacharia BE, Sisti MB. Frailty in geriatric glioblastoma patients: A predictor of operative morbidity and outcome. World Neurosurg. 2016. 89: 362-7
10. Bloom DE, Canning D, Lubet A. Global population aging: Facts, challenges, solutions and perspectives. Daedalus. 2015. 144: 80-92
11. Gerhardt J, Bette S, Janssen I, Gempt J, Meyer B, Ryang YM. Is eighty the new sixty? Outcomes and complications after lumbar decompression surgery in elderly patients over 80 years of age. World Neurosurg. 2018. 112: e555-60
12. Heiland DH, Haaker G, Watzlawick R, Delev D, Masalha W, Franco P. One decade of glioblastoma multiforme surgery in 342 elderly patients: What have we learned?. J Neurooncol. 2018. 140: 385-91
13. Huntley AL, Chalder M, Shaw AR, Hollingworth W, Metcalfe C, Richard J. A systematic review to identify and assess the effectiveness of alternatives for people over the age of 65 who are at risk of potentially avoidable hospital admission. BMJ Open. 2017. 7: e016236
14. Hussain I, Hartley BR, McLaughlin L, Reiner AS, Laufer I, Bilsky MH. Surgery for metastatic spinal disease in octogenarians and above: Analysis of 78 patients. Global Spine J. 2023. 13: 1481-9
15. Kanasi E, Ayilavarapu S, Jones J. The aging population: Demographics and the biology of aging. Periodontology. 2016. 72: 13-8
16. Lewis PR, Dunne CE, Wallace JD, Brill JB, Calvo RY, Badiee J. Routine neurosurgical consultation is not necessary in mild blunt traumatic brain injury. J Trauma Acute Care Surg. 2017. 82: 776-80
17. Macki MF, Fakih M, Rubinfeld I, Chang V, Walters BC. The impact of different postgraduate year training in neurosurgery residency on 30-day postoperative outcomes. Neurosurgery. 2019. 84: 778-87
18. Maldaner NS, Sarnthein J, Bozinov O, Regli L, Neidert MC. Neurosurgery in octogenarians: A prospective study of perioperative morbidity, mortality, and complications in elderly patients. World Neurosurg. 2018. 110: e287-95
19. Munro PT, Smith RD, Parke TR. Effect of patients’ age on management of acute intracranial haematoma: Prospective national study. BMJ. 2002. 325: 1001
20. Peduzzi PC, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996. 49: 1373-9
21. Rabinstein AA, Atkinson JL, Wijdicks EF. Emergency craniotomy in patients worsening due to expanded cerebral hematoma: To what purpose?. Neurology. 2002. 58: 1367-72
22. Reponen ET, Tuominen H, Hernesniemi J, Korja M. Modified Rankin scale and short-term outcome in cranial neurosurgery: A prospective and unselected cohort study. World Neurosurg. 2016. 91: 567-73.e7
23. Rockwood KS, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005. 173: 489-95
24. Sarnthein J, Staartjes VE, Regli L. Neurosurgery outcomes and complications in a monocentric 7-year patient registry. Brain Spine. 2022. 2: 100860
25. Shlobin NA, Kedda J, Wishart D, Garcia RM, Rosseau G. Surgical management of chronic subdural hematoma in older adults: A systematic review. J Gerontol Ser A Biol Sci Med Sci. 2021. 76: 1454-62
26. Soong JT, Poots AJ, Bell D. Finding consensus on frailty assessment in acute care through Delphi method. BMJ Open. 2016. 6: e012904
27. Teasdale GM, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow coma scale at 40 years: Standing the test of time. Lancet Neurol. 2014. 13: 844-54
28. Tomlinson SB, Piper K, Kimmell KT, Vates GE. Preoperative frailty score for 30-day morbidity and mortality after cranial neurosurgery. World Neurosurg. 2017. 107: 959-65
29. Wang MY, Widi G, Levi AD. The safety profile of lumbar spinal surgery in elderly patients 85 years and older. Neurosurg Focus. 2015. 39: E3
30. Weaver DJ, Malik AT, Jain N, Yu E, Kim J, Khan SN. The modified 5-item frailty index: A concise and useful tool for assessing the impact of frailty on postoperative morbidity following elective posterior lumbar fusions. World Neurosurg. 2019. 124: e626-32
31. Young JB, Badgery-Parker T, Dobbins T, Jorgensen M, Gibbs P, Faragher I. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage. 2015. 49: 258-64
32. Youngerman BE, Neugut AI, Yang N, Hershman DL, Wright JD, Bruce JN. The modified frailty index and 30-day adverse events in oncologic neurosurgery. J Neurooncol. 2018. 136: 197-206