- Department of Neurosurgery, The University of Tokyo, Bunkyo-ku, Japan
- Department of Neurosurgery, Teikyo University School of Medicine, Itabashi-ku, Tokyo, Japan
- Department of Neurosurgery, Asama General Hospital, Nagano, Japan.
Correspondence Address:
Satoshi Koizumi, Department of Neurosurgery, The University of Tokyo, Bunkyo-ku, Tokyo, Japan.
DOI:10.25259/SNI_460_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yudai Hirano1, Satoshi Koizumi1, Masaaki Shojima2, Osamu Ishikawa3, Satoshi Kiyofuji1, Motoyuki Umekawa1, Nobuhito Saito1. Double-catheter technique for the embolization of recurrent cerebral aneurysms: A single-center experience. 04-Aug-2023;14:273
How to cite this URL: Yudai Hirano1, Satoshi Koizumi1, Masaaki Shojima2, Osamu Ishikawa3, Satoshi Kiyofuji1, Motoyuki Umekawa1, Nobuhito Saito1. Double-catheter technique for the embolization of recurrent cerebral aneurysms: A single-center experience. 04-Aug-2023;14:273. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12487
Abstract
Background: Recurrent cerebral aneurysms have complex shapes and are often technically challenging to treat with a single microcatheter. This study evaluates the clinical characteristics and treatment outcomes of patients who received double-catheter coil embolization for recurrent cerebral aneurysms.
Methods: Patients who underwent double-catheter coil embolization at our institution between April 2011 and March 2022 for recurrent aneurysms were included in the study. Baseline characteristics, course to recurrence, details of the procedures, and outcomes after endovascular treatment were retrospectively analyzed based on past medical records.
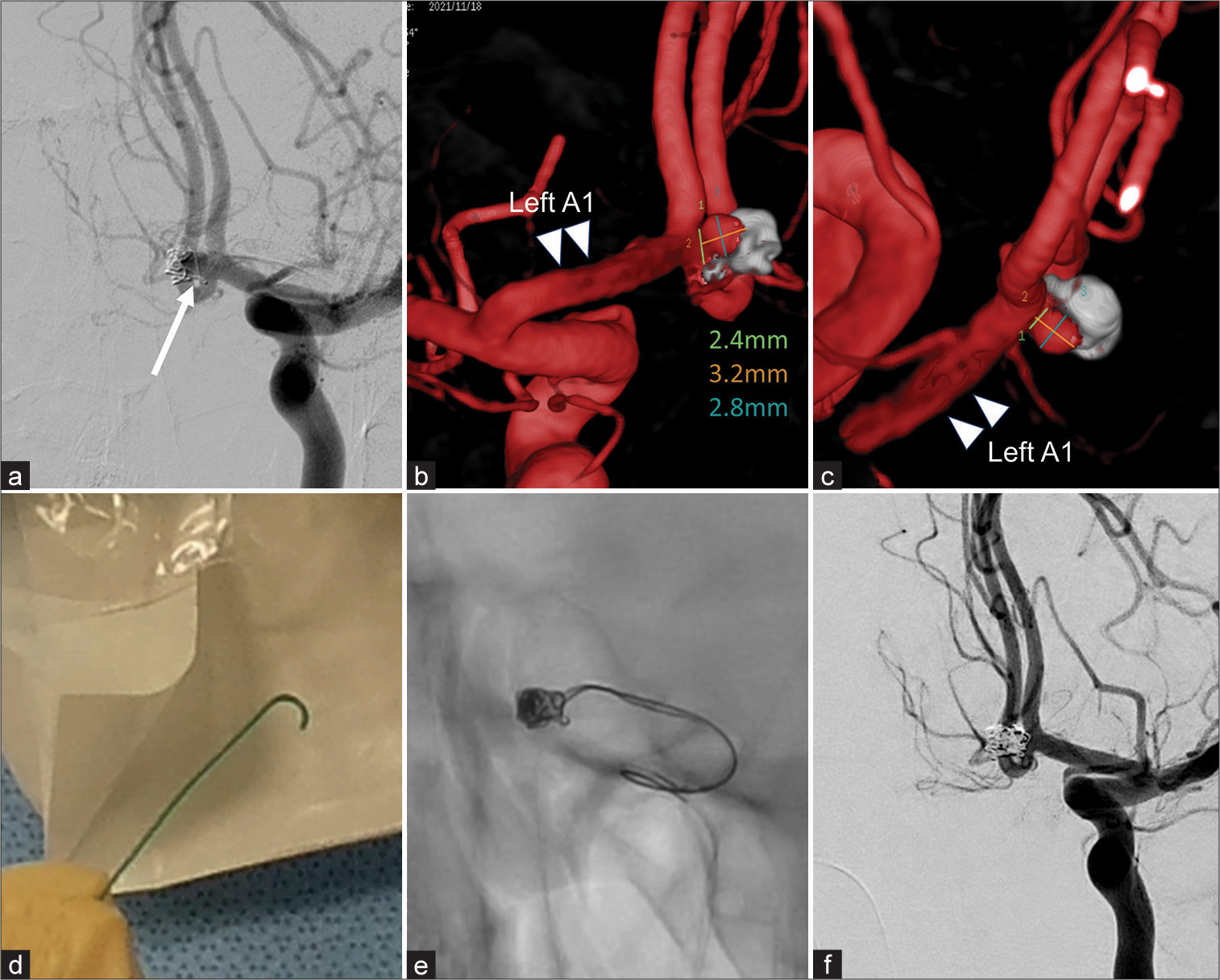
Results: Eight patients with recurrent aneurysms were treated with the double-catheter technique. One patient had a subarachnoid hemorrhage due to a rupture of a recurrent aneurysm and the others had radiological recurrence during follow-up. The initial treatment for the aneurysm was clipping in one case and coiling in seven cases. All the aneurysms were located at bifurcation sites. During retreatment, balloon remodeling technique was used in five cases. Angiographic features immediately after the treatment included complete occlusion in one case, neck remnant in three cases, and dome filling in four cases. There were no procedure-related severe complications, besides preexisting oculomotor nerve palsy due to the mass effect of the aneurysm worsened in one patient. The mean follow-up period after retreatment was 4.3 years. There was one case of recurrence after retreatment in which additional endovascular coiling was necessary.
Conclusion: This study demonstrated that the double-catheter technique could be a safe and useful treatment option for patients with recurrent aneurysms at bifurcation sites.
Keywords: Aneurysm recurrence, Coiling, Endovascular treatment, Intracranial aneurysm
INTRODUCTION
Rebleeding of a target aneurysm after treatment causes severe outcomes.[
The purpose of this study is to investigate the baseline characteristics, endovascular procedure, and long-term results of coil embolization for recurrent cerebral aneurysms using the double-catheter technique, presenting radiological images and an intraoperative video of a representative case, and to evaluate its efficacy and safety.
MATERIALS AND METHODS
Subjects
This study was approved by the Institutional Review Board of our hospital (#2231). The subjects of this study were patients with recurrent cerebral aneurysms who underwent coil embolization using the double-catheter technique at our institution between 2011 and 2022. The criteria for the treatment of recurrent cerebral aneurysms at our institution are as follows: (1) subarachnoid hemorrhage or (2) increased size on radiological follow-up with magnetic resonance angiography (MRA) or digital subtraction angiography (DSA). Cases of recurrent cerebral aneurysms treated with endovascular therapy were screened. The patients in which coil embolization was performed using the double-catheter technique were extracted. Medical records, operative courses, and radiological data were retrospectively analyzed.
Endovascular procedures
In patients with unruptured aneurysms, in principle, a single antiplatelet agent (aspirin 100 mg or clopidogrel 75 mg) was initiated 3 weeks before surgery, and administration of the agent was adjusted according to each case. In patients with subarachnoid hemorrhage, a loading dose of aspirin (200 mg) was applied through a nasogastric tube after arterial sheath insertion. Endovascular procedures were performed under general anesthesia. After placing a sheath through the right femoral artery, heparin was injected systemically to maintain an activated clotting time of 1.5–2.5 times the initial value. The double-microcatheter technique was mainly used for wide-neck, bifurcation-type aneurysms, where it was difficult to achieve sufficient coil embolization with a single microcatheter. This method was applied in cases where the diameter of the parent artery was sufficiently large (approximately >2 mm) and the arteriosclerosis was not severe enough to interfere with the guidance of the microcatheter. The aneurysm size was measured in three directions using three-dimensional rotational angiography (3DRA). The sizes of the first and second coils were investigated retrospectively. In the results section, these values are expressed as mean ± standard deviation, unless otherwise stated. In cases where the coil protruded into the parent vessel during coil insertion, the balloon remodeling technique was used selectively.[
Management after coil embolization
Postoperative neurological status was assessed immediately after the treatment in the intensive care unit and at a discharge. Head computed tomography (CT) and magnetic resonance imaging/angiography were performed on postoperative day 1 to check hemorrhage and cerebral infarction. To prevent ischemic complications, a single antiplatelet drug (aspirin 100 mg daily or clopidogrel 75 mg daily) was continued for 1 month after the treatment. Follow-up after an endovascular treatment was performed at the outpatient clinic at 3 months, 6 months, and 1 year. In CT angiography and MRA, accurate evaluation of the recurrence is difficult due to coil artifacts; therefore, we checked the recurrence by DSA as long as the patient’s condition permitted.
RESULTS
Of 281 endovascular treatments for cerebral aneurysms performed during the study period, 19 (6.8%) were for recurrent aneurysms. The double-catheter technique was used in eight of the 19 patients treated (42%), which were evaluated in the following analyses. Of the remaining 11 patients, six were treated with a simple technique, two with a neck-bridging stent, and two with a flow diverter (FD). In one patient, a catheter was difficult to navigate, and the treatment was unsuccessful. The patients’ baseline characteristics are described in
Representative case (Case 8)
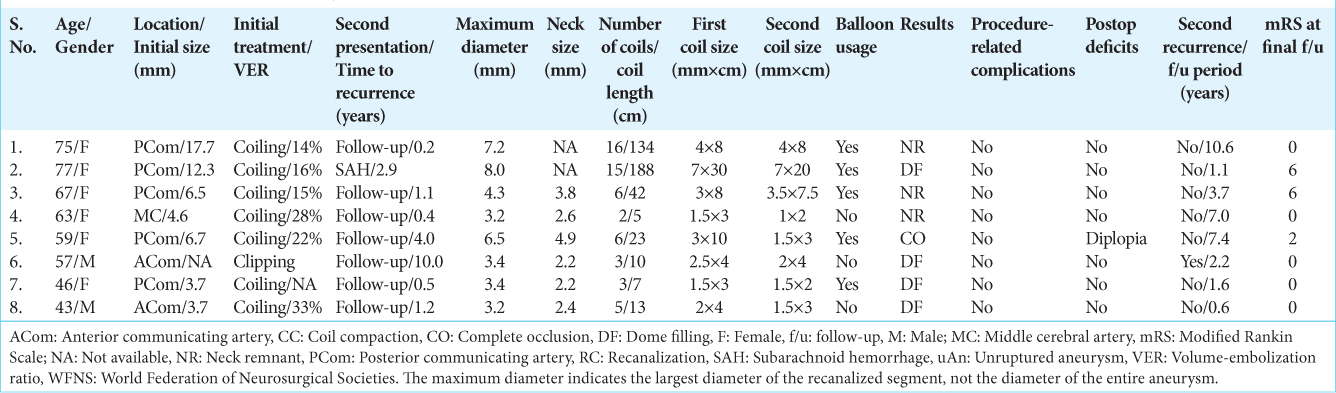
A 43-year-old man, who underwent endovascular coiling using the balloon remodeling technique for subarachnoid hemorrhage (World Federation of Neurosurgical Societies grade 2) due to a rupture of an ACoA aneurysm with a diameter of 3.7 mm, was found to have a recurrent aneurysm on DSA 1 year after the initial treatment [
Figure 1:
Pre and postoperative images of a representative case (Case 8) (a) Left internal carotid angiography shows a recurrent aneurysm located in the anterior communicating artery. One loop of the preexisting coil (arrow) protrudes at the neck. (b,c) Three-Dimensional rotational angiography shows the direction of protrusion of the aneurysm relative to the anterior cerebral artery A1 (double arrowhead) from the posterior (b) and superior (c) view. (d) The tip of the Headway 17 microcatheter is formed at a double angle. (e) The initial coil inserted from Headway17 was unstable, and the second coil was added from the other SL-10 preshape J microcatheter before detaching the first coil. (f) Postoperative digital subtraction angiography (DSA) shows slight body filling without interfering with the parent vessel.
Video 1
DISCUSSION
This study focused on endovascular treatment using the double microcatheter technique for recurrent aneurysms. There were no cases of intraoperative rupture, while postoperative exacerbation of oculomotor nerve palsy was observed in one patient, but no new neurological deficits were identified in the other cases. With a median follow-up of 3.0 years, 7 of 8 cases (87.5%) were recurrence-free.
Aneurysms have a high risk of developing subarachnoid hemorrhage due to re-enlargement[
The rates of complete occlusion immediately after surgery and long-term occlusion are higher when adjunct with neck-bridging stents or FDs than simple coiling for both primary and recurrent cerebral aneurysms.[
The recanalized cavity inside preexisting coils or clips has an irregular shape, making endovascular embolization of recurrent aneurysms technically challenging, and it is generally difficult to form a stable frame in the initial stage of coiling. As shown in the representative case, it is sometimes necessary to use short and soft coils initially to hold them inside the aneurysm. However, these coils are unstable and there is a risk of coil protrusion into the parent artery when inserting subsequent coils. Considering the geometric nature of recurrent aneurysms, the advantage of the double-microcatheter method is the formation of a more stable frame before detaching the first coil. Technically, it is important to place the two microcatheters at different positions inside the target aneurysm. Usually, the first microcatheter is placed near the neck and the other is placed in the center. Regarding the selection of the first and second coil in our strategy, it is important to choose the small-diameter coil according to the maximal length of the recanalized aneurysm as the first coil, and not to decrease the diameter of the second coil too much compared to the first one. Consequently, a stable frame within the recanalized cavity can be safely created using the first two coils.
The disadvantages of this technique include the complexity of the procedure and the potential difficulty in removing the microcatheter. The technical tips are as follows. In the beginning, the 1st and 2nd coils are stabilized by alternately moving them in and out and entangling them with each other. If knots of microcatheters are formed at this time, the two microcatheters were pulled out with their coils. This allows the coil embolization to be performed again from the beginning. It is important to insert two microcatheters from the same guiding or distal access catheter to achieve this bailout. On the other hand, the third and subsequent coils should not be forcibly intertwined with the previous coils. We simply pushed the coils through the microcatheter and repeated detaching them as soon as they settled within the aneurysm. This rarely caused knots in the each microcatheter, making it difficult to remove. In addition, it is sometimes difficult to distinguish between the markers of the two microcatheters, which can be overcome by frequently changing the angle of the flat panels.
This study has some limitations. First, this was a retrospective study with a small sample size. Second, all recurrent aneurysms coiled with the double-catheter technique were included in the study. Because the morphological characteristics of recurrent aneurysm after coiling and clipping may not be strictly the same, future analyses should be classified according to the method of initial treatment. Third, we did not compare patient backgrounds and treatment outcomes between the double microcatheter technique and other techniques used. Fourth, endovascular procedures and criteria for adoption of treatment methods have not been standardized because multiple operators have performed endovascular treatment during the target period. Thus, there are no strict criteria to distinguish between simple catheter and double catheter techniques in this study period. Fifth, novel remodeling devices for bifurcation aneurysms, such as WEB (Microvention, USA) and PulseRider (Cerenovus, USA), were not used at our center during the study period.
Despite these limitations, our study demonstrated the efficacy of the double-catheter technique in treating recurrent aneurysms. Our technique can be a reasonable option to simultaneously minimize invasiveness and reduce the recurrence rate. The future studies are warranted to investigate appropriate management strategies for recurrent cerebral aneurysms.
CONCLUSION
The double-catheter technique is a safe and useful treatment option for patients with recurrent cerebral aneurysms at bifurcation sites. The technique can assure the long-term occlusion of the target aneurysm with relatively low perioperative complication rate.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Baxter BW, Rosso D, Lownie SP. Double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. AJNR Am J Neuroradiol. 1998. 19: 1176-8
2. CARAT Investigators. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006. 37: 1437-42
3. Ciccio G, Robert T, Smajda S, Fahed R, Desilles JP, Redjem H. Double stent assisted coiling of intracranial bifurcation aneurysms in Y and X configurations with the Neuroform ATLAS stent: Immediate and mid term angiographic and clinical follow-up. J Neurointerv Surg. 2019. 11: 1239-42
4. Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999. 212: 348-56
5. De Keukeleire K, Vanlangenhove P, Defreyne L. Evaluation of a neck-bridge device to assist endovascular treatment of wide-neck aneurysms of the anterior circulation. AJNR Am J Neuroradiol. 2008. 29: 73-8
6. Froelich JJ, Cheung N, de Lange JA, Monkhorst J, Carr MW, DeLeacy R. Residuals, recurrences and re-treatment after endovascular repair of intracranial aneurysms: A retrospective methodological comparison. Interv Neuroradiol. 2020. 26: 45-54
7. Giannotta SL, Litofsky NS. Reoperative management of intracranial aneurysms. J Neurosurg. 1995. 83: 387-93
8. Kim DJ, Kim BM, Park KY, Ihm EH, Baek JH, Kim DI. Coil embolization of overwide and undertall small intracranial aneurysms with double microcatheter technique. Acta Neurochir (Wien). 2014. 156: 839-46
9. Kwon OK, Kim SH, Kwon BJ, Kang HS, Kim JH, Oh CW. Endovascular treatment of wide-necked aneurysms by using two microcatheters: Techniques and outcomes in 25 patients. AJNR Am J Neuroradiol. 2005. 26: 894-900
10. Li YD, Li MH, Gao BL, Fang C, Cheng YS, Wang W. Endovascular treatment of recurrent intracranial aneurysms with re-coiling or covered stents. J Neurol Neurosurg Psychiatry. 2010. 81: 74-9
11. Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg. 1989. 70: 556-60
12. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009. 8: 427-33
13. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005. 366: 809-17
14. Phan K, Huo YR, Jia F, Phan S, Rao PJ, Mobbs RJ. Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracranial aneurysms. J Clin Neurosci. 2016. 31: 15-22
15. Pierot L, Cognard C, Anxionnat R, Ricolfi F, CLARITY Investigators. Remodeling technique for endovascular treatment of ruptured intracranial aneurysms had a higher rate of adequate postoperative occlusion than did conventional coil embolization with comparable safety. Radiology. 2011. 258: 546-53
16. Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ. Stent-assisted coiling of intracranial aneurysms: Clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010. 41: 110-5
17. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003. 34: 1398-403
18. Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol. 2007. 28: 1755-61
19. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001. 32: 1998-2004
20. Shapiro M, Babb J, Becske T, Nelson PK. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: A literature review. AJNR Am J Neuroradiol. 2008. 29: 1777-81
21. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ. The barrow ruptured aneurysm trial: 6-year results. J Neurosurg. 2015. 123: 609-17