- Geisel School of Medicine, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, USA
- Department of Emergency Medicine, University of Iowa Hospitals and Clinics, Iowa City, USA
- Department of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, USA
- Dartmouth Cancer Center, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, USA
- Department of Neurosurgery, New York University Langone Health, New York, USA.
Correspondence Address:
George Zanazzi, Department of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, NH 03756, USA.
DOI:10.25259/SNI_3_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alice Liu1, Joshua S. Bauer2, Chun-Chieh Lin3,4, Geoff Appelboom5, George Zanazzi3,4. Dural composite hemangioendothelioma: The first intracranial case. 23-Feb-2024;15:55
How to cite this URL: Alice Liu1, Joshua S. Bauer2, Chun-Chieh Lin3,4, Geoff Appelboom5, George Zanazzi3,4. Dural composite hemangioendothelioma: The first intracranial case. 23-Feb-2024;15:55. Available from: https://surgicalneurologyint.com/surgicalint-articles/12765/
Abstract
Background: Composite hemangioendothelioma (CHE) is a rare, locally aggressive neoplasm of intermediate malignant potential. It is composed of a mixture of vascular tumors with a predilection for the dermis and subcutis of the extremities.
Case Description: In this report, we describe a 41-year-old man who presented with a 2-month history of headache, dizziness, and intermittent seizures. Magnetic resonance imaging showed a hemorrhagic, multilobulated, and dural-based mass with extension into the calvarium. The mass measured 10.3 × 4.8 × 4 cm along the interhemispheric fissure and encased the superior sagittal sinus. Excision was performed, and histopathologic examination revealed a heterogeneous mixture of vascular components consisting of epithelioid hemangioendothelioma, retiform hemangioendothelioma, and hemangioma. This is the first report of a primary intracranial CHE.
Conclusion: The spectrum of mesenchymal neoplasms within the cranium expands to encompass CHE.
Keywords: Dura, Epithelioid hemangioendothelioma, Falx cerebri, Retiform hemangioendothelioma, Vascular neoplasm
INTRODUCTION
Hemangioendotheliomas are vascular neoplasms that exhibit an intermediate level of malignancy and display histopathological characteristics that fall between those of a hemangioma and an angiosarcoma. They are located predominantly in the skin and subcutaneous tissue of the distal extremities. Although they can be locally aggressive, they rarely metastasize. They encompass a diverse spectrum of histologic types, including epithelioid hemangioendothelioma (EHE), retiform hemangioendothelioma (RHE), papillary intralymphatic angioendothelioma, pseudomyogenic hemangioendothelioma, kaposiform hemangioendothelioma (KHE), and composite hemangioendothelioma (CHE).[
CHE refers to a rare neoplasm of intermediate malignant potential composed of two or more of the following tumors: EHE, RHE, papillary intralymphatic angioendothelioma, KHE, and angiosarcoma. Well-differentiated vascular lesions, such as hemangiomas and arteriovenous malformations, may also be found in CHE. Very few cases of CHE have been described in the literature, and they usually involve the skin or soft tissue.[
CASE HISTORY
The patient is a 41-year-old right-handed man who presented to an ophthalmologist with diplopia for the prior two months. He had also been experiencing intractable headaches, dizziness, and intermittent seizures manifesting as shaking of his right leg, with each episode lasting 2–3 min. These symptoms had steadily progressed, prompting him to seek medical consultation. He denied any previous similar episodes or any significant medical history. Although there was full vision of the left eye, there was right nasal hemianopsia with superior field cuts. He was sent to the emergency department for evaluation of bilateral papilledema.
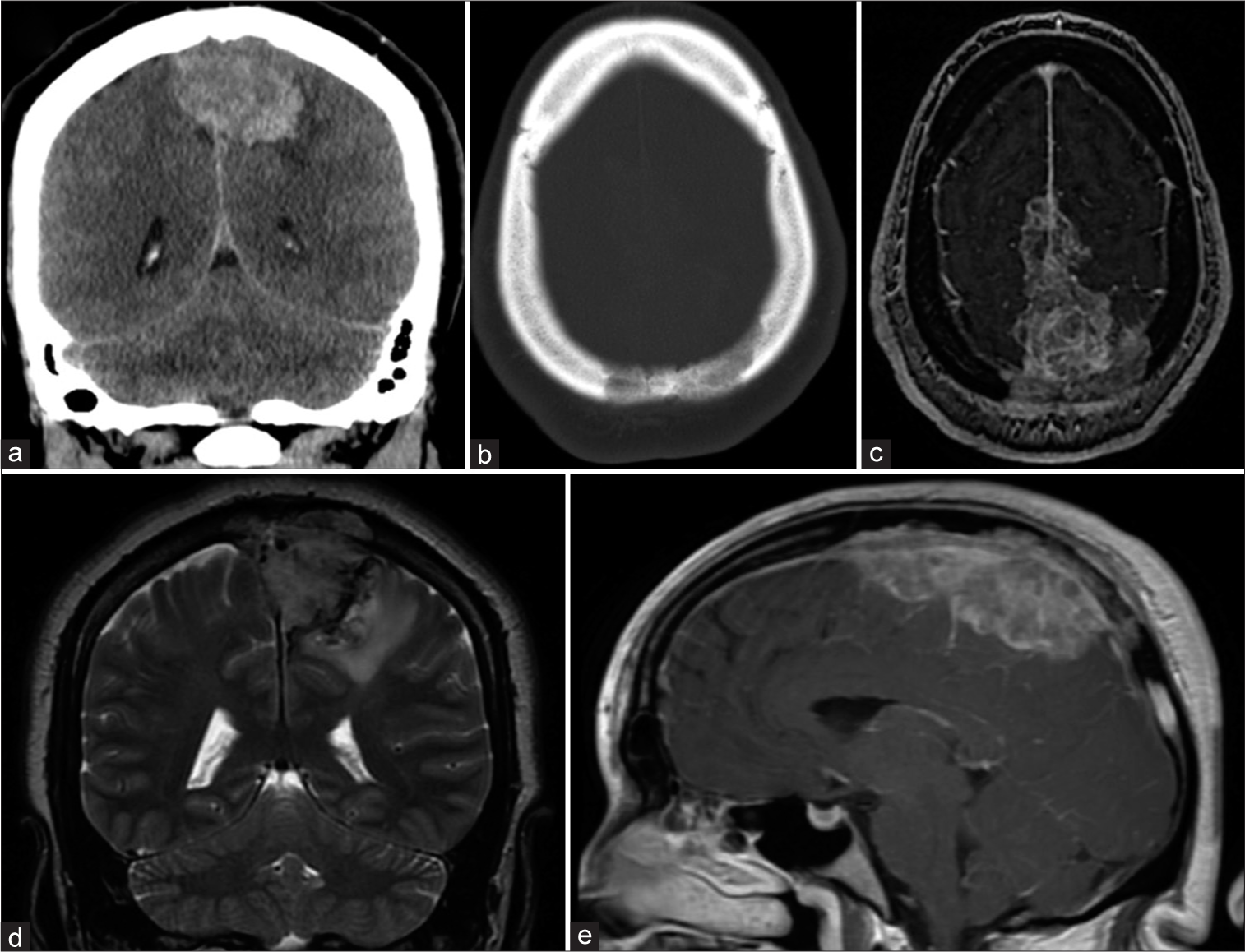
A baseline head computed tomography (CT) scan showed a lobulated hyperdense soft-tissue mass along the interhemispheric fissure and superior sagittal sinus (SSS) [
Figure 1:
Baseline noncontrast computed tomography and magnetic resonance imaging (MRI) images of the brain. (a) Coronal view shows an interhemispheric mass with left-sided mass effect. (b) Axial view using bone window reveals lytic involvement of calvarium. (c) Axial T2-weighted MRI shows superior sagittal sinus involvement. (d) Coronal T2-weighted MRI reveals tumor extension through the calvarium. (e) Sagittal T1-weighted MRI post-gadolinium shows tumor enhancement.
The patient was sent for a formal angiography procedure after identifying possible SSS occlusion on MRI. There was a postangiography procedure diagnosis of partial occlusion of the posterior second third of the SSS with collateral drainage primarily to the left side through cortical veins (not shown). Artery embolization was not feasible, given the location of the neoplasm relative to the SSS. Based on the clinical presentation and neuroimaging, the decision was made to take the patient for a bilateral frontoparietal craniectomy for tumor resection. Gross appearance during surgery revealed a hard, fibrous mass with marked vascularity (not shown). The neoplasm seemed to originate from dura overlying the SSS, tracking along the interhemispheric fissure and extending to the surrounding cranium (not shown). Creating planes among the mass and parenchyma was difficult due to the adherent nature of the neoplasm. Gross total resection was limited by the location of the neoplasm involving a substantial part of the SSS and the anterior paracentral lobule. Once larger portions of neoplasm located away from eloquent areas were excised, careful attention was turned to debulking the remaining neoplasm with the use of the Cavitron Ultrasonic Surgical Aspirator. The patient experienced an uncomplicated 2-week postoperative course before being sent to inpatient rehabilitation and then Gamma Knife radiotherapy for residual tumor.
Pathology
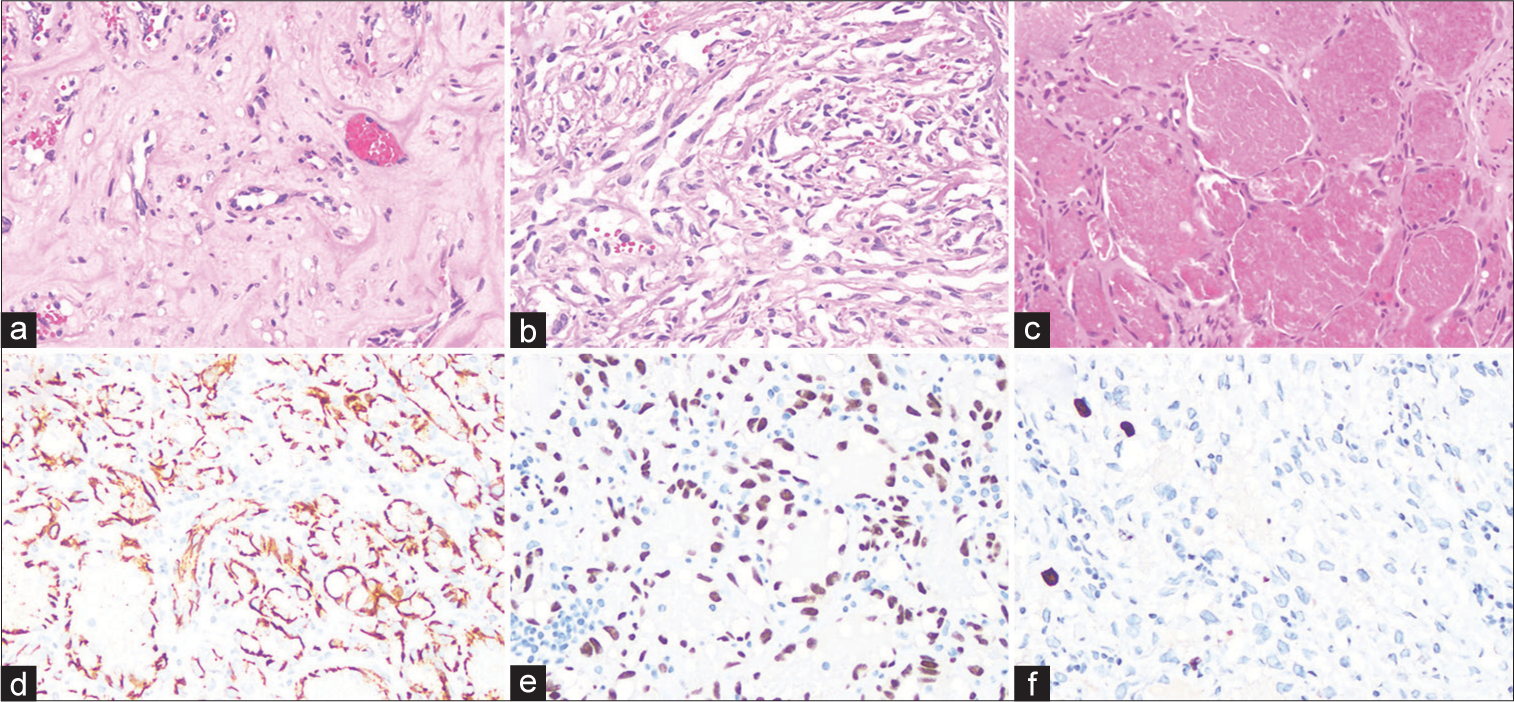
Histopathological examination of the neoplasm showed an admixture of different components with focal infiltration into the dura and bone. Approximately 30% of the neoplasm had abundant eosinophilic cytoplasm, nuclear atypia, and intracytoplasmic vacuoles with occasional hyalinized stroma, consistent with EHE [
Figure 2:
Histologic features of the intracranial composite hemangioendothelioma. (a) Hematoxylin and eosin (H&E)-stained section shows an area of epithelioid hemangioendothelioma, with cells containing intracytoplasmic vacuoles embedded in a hyaline stromal matrix. (b) H&E-stained section shows an area of retiform hemangioendothelioma, with abundant arborizing vascular channels. (c) H&E-stained section shows an area of hemangioma, with dilated vascular channels lined by oval nuclei without atypia. (d) An anti-CD31 antibody reveals abundant endothelial cells in this neoplasm. (e) An anti-ERG antibody confirms the abundant endothelial cells in this neoplasm. (f) The Ki-67 proliferation index is low, with up to 3.6% of cells labeled with this antibody.
DISCUSSION
Here, we present the first case report of a primary intracranial CHE in a 41-year-old man who presented with headache, dizziness, and seizures. On initial presentation and baseline CT and MRI [
First described in 2000, CHE is a rare, intermediate grade slowly growing vascular lesion that usually presents as an erythematous nodule in the dermis or subcutaneous tissue of the extremities. Less than 100 cases have been reported in the literature [
In comparison to conventional angiosarcoma, most CHEs exhibit less aggressive behavior. However, adjuvant therapy may be warranted in cases of subtotal resection, given the rate of local recurrence and malignant potential.[
Since EHE is a common component of CHE,[
The other common component in CHE, seen in our patient’s tumor, is RHE.[
Other hemangioendotheliomas may occur within the cranium. Single cases of intracranial pseudomyogenic hemangioendothelioma[
CONCLUSION
We present the clinicopathologic features of a primary intracranial CHE in an adult man. The large hemorrhagic mass appeared to originate from the falx cerebri and infiltrate the overlying calvarium. Histopathologic examination of the resected neoplasm revealed a mixture of EHE, RHE, and hemangioma. Primary intracranial CHE has not been previously reported in the literature. Based on a review of published composite hemangioendotheliomas elsewhere in the body, this neoplasm should be managed with aggressive surgical resection when possible.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Research Committee of University of Helsinki and with the 1964 Declaration of Helsinki and its amendments or comparable ethical standards.
Declaration of patient consent
Patient’s consent was not required as as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aditya GS, Santosh V, Yasha TC, Shankar SK. Epithelioid and retiform hemangioendothelioma of the skull bone--report of four cases. Indian J Path Micro. 2003. 46: 645-9
2. Ahmed S, Epari S, Shah M, Rao KS. Epithelioid hemangioendothelioma of sphenoid bone: A case report of an unusual case. Neurol India. 2012. 60: 344-6
3. Aniba K, Laghmari M, Lmejjati M, Ghannane H, Ait Benali S. A tragical paediatric case history of intraorbital and intracranial epithelioid hemangioendothelioma. Case Rep Neurol Med. 2012. 2012: 396097
4. Antonescu CR, Dickson BC, Sung YS, Zhang L, Suurmeijer AJ, Stenzinger A. Recurrent YAP1 and MAML2 gene rearrangements in retiform and composite hemangioendothelioma. Am J Surg Path. 2020. 44: 1677-84
5. Aydingöz IE, Demirkesen C, Serdar ZA, Mansur AT, Yaşar S, Aslan C. Composite haemangioendothelioma with lymph-node metastasis: An unusual presentation at an uncommon site. Clin Exp Derm. 2009. 34: e802-6
6. Aznar AO, Vidal FR, Mendez EV, de Grassa BI. Intracranial hemangioendotheliomas. Int J Neuroradiol. 1998. 4: 366-72
7. Baehring JM, Dickey PS, Bannykh SI. Epithelioid hemangioendothelioma of the suprasellar area: A case report and review of the literature. Arch Pathol Lab Med. 2004. 128: 1289-93
8. Balko J, Ozaniak A, Krskova L, Strizova Z, Lischke R, Zamecnik J. Patient with composite haemangioendothelioma containing angiosarcoma-like areas in the setting of congenital lymphoedema mimicking Stewart-Treves syndrome: A case report. Diagnostic Path. 2023. 18: 76
9. Barger J, Tanweer O, Liechty B, Snuderl M, Jafar JJ. Suprasellar epithelioid hemangioendothelioma: Case report and review of the literature. Surg Neurol Int. 2016. 7: S596-602
10. Batista KP, Gómez GL, Quintana EM, Astudillo A, Fernandez-Vega I, Fernandez BA. Giant cranionasal epithelioid hemangioendothelioma with invasive growth pattern mimicking a skull base chondrosarcoma. Contemp Oncol. 2018. 22: 118-23
11. Bhat A, Chowdappa V. Composite hemangioendothelioma: Report of a rare case. J Clin Diagn Res. 2016. 10: ED01-03
12. Biagioli M, Sbano P, Miracco C, Fimiani M. Composite cutaneous haemangioendothelioma: Case report and review of the literature. Clin Exp Derm. 2005. 30: 385-7
13. Bui CM, Balzer B. Multiply recurrent composite hemangioendothelioma of penis with histologic progression to high-grade features. Dermpath. 2023. 10: 41-5
14. Cai Y, Li J, Yang W, Zhang N, Sun H, Zhang W. Case report: Congenital intracranial Kaposiform hemangioendothelioma treated with surgical resection. Front Surg. 2022. 9: 831190
15. Cakir E, Demirag F, Gulhan E, Oz G, Tastepez I. Mediastinal composite hemangioendothelioma. A rare tumor at an unusual location. Tumori. 2009. 95: 98-100
16. Chan YL, Ng HK, Poon WS, Cheung HS. Epithelioid hemangioendothelioma of the brain: A case report. Neuroradiology. 2001. 43: 848-50
17. Chang JM, Kwon BJ, Han MH, Kang HS, Chang KH. Kaposiform hemangioendothelioma arising from the internal auditory canal. Am J Neuroradiol. 2006. 27: 931-3
18. Chen TC, Gonzalez-Gomez I, Gilles FH, McComb JG. Pediatric intracranial hemangioendotheliomas: Case report. Neurosurgery. 1997. 40: 410-4
19. Chen YL, Chen WX, Wang J, Jiang Y. Composite hemangioendothelioma on the neck. Kaohsiung J Med Sci. 2012. 28: 564-5
20. Cheuk W, Shum KS, Ng WK, Chan JK. Composite hemangioendothelioma with neuroendocrine marker expression: Report of a “paraganglioma-like” paravertebral case. Int J Surg Path. 2020. 28: 759-63
21. Chin S, Kim J, Jung MJ, Kim MJ, Moon A, Kim HK. Intramuscular composite hemangioendothelioma: Case report of an unusual tumor in an unusual location. Int J Clin Exp Path. 2020. 13: 1421-5
22. Cho WS, Kim SK, Park SH, Cho BK. Intracranial kaposiform hemangioendothelioma: Proposal of a new malignant variant. J Neurosurg Pediatr. 2009. 3: 147-50
23. Chow LT, Chow WH, Fong DT. Epithelioid hemangioendothelioma of the brain. Am J Surg Path. 1992. 16: 619-25
24. Das S, Deora H, Rao S, Kandregula S, Narayana SM. Intracranial kaposiform hemangioendothelioma presenting as epistaxis: A rare case report with review of literature. Child Nerv Syst. 2021. 37: 2057-62
25. Dermawan JK, Westra WH, Antonescu CR. Recurrent PTBP1::MAML2 fusions in composite hemangioendothelioma with neuroendocrine differentiation: A report of two cases involving neck lymph nodes. Genes Chrom Cancer. 2022. 61: 187-93
26. Drazin D, Gandhi R, Slodkowska E, Boulos AS. Epithelioid hemangioendothelioma of the mastoid: Resection for recurrence and adjuvant radiation with 8-year follow-up. Case Rep Surg. 2013. 2013: 469201
27. Drut R, Sapia S, Gril D, Velasco JC, Drut RM. Nonimmune hydrops fetalis, hydramnios, microcephaly, and intracranial meningeal hemangioendothelioma. Pediatr Pathol. 1993. 13: 9-13
28. Fasolis M, Iaquinta C, Montesco MC, Garzino-Demo P, Tosco P, Tanteri G. Composite hemangioendothelioma of the oral cavity: Case report and review of the literature. Head Neck. 2008. 30: 974-9
29. Fernandes AL, Ratilal B, Mafra M, Magalhaes C. Aggressive intracranial and extracranial epithelioid hemangioendothelioma: A case report and review of the literature. Neuropathology. 2006. 26: 201-5
30. Fryer JA, Biggs MT, Katz IA, Brazier DH, Shakespeare TP. Intracranial epithelioid hemangioendothelioma arising at site of previously excised atypical meningioma. Pathology. 1998. 30: 95-9
31. Fukunaga M, Suzuki K, Saegusa N, Folpe AL. Composite hemangioendothelioma: Report of 5 cases including one with associated Maffucci syndrome. Am J Surg Pathol. 2007. 31: 1567-72
32. Gok S, Berkman MZ, Baykara E. Composite hemangioendothelioma settled in the paraspinal region: A rare case report. Turk Neurosurg. 2020. 30: 299-302
33. Golash A, Strang FA, Reid H. Intracranial hemangioendothelioma mimicking a meningioma. Br J Neurosurg. 1999. 13: 594-7
34. Gündoğan BD, Çıtak EÇ, Sağcan F, Esen K, Yıldız A, Arpacı RB. Temporal bone hemangioendothelioma as a rare vascular tumor in childhood: Case report and review of the literature. Turk J Pediatr. 2020. 62: 843-50
35. Hamlat A, Casallo-Quilliano C, Saikali S, Lesimple T, Brassier G. Epithelioid hemangioendothelioma of the infundibular-hypothalamic region: Case report and literature review. J Neurooncol. 2004. 67: 361-6
36. Han DS, Ahn DW, Lee JA, Lee MS, Chang MS. Gastric composite hemangioendothelioma, manifesting iron-deficiency anemia and endoscopically mimicking EGC type I. Korean J Int Med. 2022. 37: 1260-1
37. Hardisson D, Prim MP, De Diego JI, Patrón M, Escribano A, Rabanal I. Kaposiform hemangioendothelioma of the external auditory canal in an adult. Head Neck. 2002. 24: 614-7
38. Hodaie M, Becker L, Teshima I, Rutka JT. Total resection of an intracerebral hemangioendothelioma in an infant. Case report and review of the literature. Pediatr Neurosurg. 2001. 34: 104-12
39. Huang W, Li L, Gao J, Kang L. A case of complex hemangioendothelioma of the liver in an infant. Rev Esp Enferm Dig. 2023. 115: 668-70
40. Hurley TR, Whisler WW, Clasen RA, Smith MC, Bleck TP, Doolas A. Recurrent intracranial epithelioid hemangioendothelioma associated with multicentric disease of liver and heart: Case report. Neurosurgery. 1994. 35: 148-51
41. Jones CM, Nieweg OE, Isaacs F, Cheung K. Composite haemangioendothelioma with neuroendocrine marker differentiation presenting as a pink-brown nodule. Australas J Dermatol. 2023. p.
42. Jung SC, Jung TY, Lee TK, Kim YJ, Baek HJ, Kim SS. Kaposiform hemangioendothelioma of skull base with Dura invasion in a pediatric patient: A case report. Child Nerv Syst. 2023. 39: 3289-94
43. Kepes JJ, Rubinstein LJ, Maw G, Burdick B. Epithelioid hemangiomas (hemangioendotheliomas) of the central nervous system and its coverings, a report of three cases. J Neuropath Exp Neurol. 1986. 45: 319
44. Kim IK, Cho HY, Jung BS, Pae SP, Cho HW, Seo JH. Retiform hemangioendothelioma in the infratemporal fossa and buccal area: A case report and literature review. J Korean Assoc Oral Maxillo Surg. 2016. 42: 307-14
45. Koh YC, Yoo H. Epithelioid hemangioendothelioma of the sphenoid bone. J Clin Neurosci. 2001. 8: 63-6
46. Koutlas IG, Oetting WS, Burns GM, Gopalakrishnan R, Antonescu CR. Whole exome sequencing identifies somatic variants in an oral composite hemangioendothelioma characterized by YAP1-MAML2 fusion. Head Neck Path. 2022. 16: 849-56
47. Kubota T, Sato K, Takeuchi H, Handa Y. Successful removal after radiotherapy and vascular embolization in a huge tentorial epithelioid hemangioendothelioma: A case report. J Neurooncol. 2004. 68: 177-83
48. Langguth P, Salehi Ravesh M, Haneya A, Both M. Composite hemangioendothelioma: The first case of a right atrioventricular pericardial tumour. Eur Heart J Case Rep. 2020. 4: 1
49. Leen SL, Clarke PM, Chapman J, Fisher C, Thway K. Composite hemangioendothelioma of the submandibular region. Head Neck Pathol. 2015. 9: 519-24
50. Li WW, Liang P, Zhao HP, Zhang YX, Liu YY, Gao JB. Composite hemangioendothelioma of the spleen with multiple metastases: CT findings and review of the literature. Medicine (Baltimore). 2021. 100: e25846
51. Liau JY, Lee FY, Chiu CS, Chen JS, Hsiao TL. Composite hemangioendothelioma presenting as a scalp nodule with alopecia. J Am Acad Dermatol. 2013. 69: e98-9
52. Linos K, Dermawan JK, Pulitzer M, Hameed M, Agaram NP, Agaimy A. Untying the Gordian knot of composite hemangioendothelioma: Discovery of novel fusions. Genes Chromosomes Cancer. 2024. 63: e23198
53. Llena JF, Hirano A, Inoue A. Vasoformative tumor of the brain--immunohistology and ultrastructure. Clin Neuropathol. 1984. 3: 155-9
54. Ma SR, Li KC, Xu YQ, Wang YM, Ma WL, Li Q. Primary epithelioid hemangioendothelioma in the clival region: A case report and literature review. Neuropathology. 2011. 31: 519-22
55. Mahmoudizad R, Samrao A, Bentow JJ, Peng SK, Bhatia N. Composite hemangioendothelioma: An unusual presentation of a rare vascular tumor. Am J Clin Pathol. 2014. 141: 732-6
56. McNab PM, Quigley BC, Glass LF, Jukic DM. Composite hemangioendothelioma and its classification as a low-grade malignancy. Am J Dermatopathol. 2013. 35: 517-22
57. Medina M, Polo R, Reyes P, Vaca M, Alonso A, Cobeta I. Imaging case of the month. Multifocal epithelioid hemangioendothelioma with massive lateral skull base involvement. Otol Neurotol. 2015. 36: e67-9
58. Miyamoto E, Seki K, Katsuragawa H, Yoshimoto Y, Ohsumi Y, Fukui T. Thoracic composite hemangioendothelioma with neuroendocrine marker expression. Surg Case Rep. 2021. 7: 249
59. Murali M, Symss N, Pande A, Chakravarthy V, Ramamurthi R. Intracranial epithelioid hemangioendothelioma. Child Nerv Syst. 2008. 24: 863-8
60. Nakamura S, Uehara M, Kobayashi S, Hasegawa H, Tanaka A, Takahashi J. Composite hemangioendothelioma in the cervical spine with kaposiform hemangioendothelioma features in an elderly patient: a case report. BMC Geriatr. 2022. 22: 952
61. Nayler SJ, Rubin BP, Calonje E, Chan JK, Fletcher CD. Composite hemangioendothelioma: A complex, low-grade vascular lesion mimicking angiosarcoma. Am J Surg Pathol. 2000. 24: 352-61
62. Nora FE, Scheithauer BW. Primary epithelioid hemangioendothelioma of the brain. Am J Surg Pathol. 1996. 20: 707-14
63. Omerhodžić I, Bilalović N, Rovčanin B, Imširović B, Suljić E, Rotim A. Primary epithelioid hemangioendothelioma in the cerebellum: Case report with reference to drastic change in the WHO classification. Acta Clin Croat. 2018. 57: 570-6
64. Ooi S, Gutman M, Xenos C, Chandra R, McLean C. Surgical considerations in a pediatric case of a large skull-base epithelioid hemangioendothelioma. Childs Nerv Syst. 2019. 35: 559-63
65. Pacheco JM, Goodman JC, Mandel J. Intracranial epithelioid hemangioendothelioma causing subacute loss of vision. Neurology. 2015. 85: 735-6
66. Palmieri G, Montella L, Martignetti A, Bianco AR. Interferon alpha-2b at low doses as long-term antiangiogenic treatment of a metastatic intracranial hemangioendothelioma: A case report. Oncol Rep. 2000. 7: 145-9
67. Parajon A, Vaquero J. Meningel intracranial epithelioid hemangioendothelioma: Case report and literature review. J Neurooncol. 2008. 88: 169-73
68. Pearl GS, Takei Y, Tindall GT, O’Brien MS, Payne NS, Hoffman JC. Benign hemangioendothelioma involving the central nervous system: “Strawberry nevus” of the neuraxis. Neurosurgery. 1980. 7: 249-56
69. Perry KD, Al-Lbraheemi A, Rubin BP, Jen J, Ren H, Jang JS. Composite hemangioendothelioma with neuroendocrine marker expression: An aggressive variant. Mod Pathol. 2017. 30: 1512
70. Phookan G, Davis AT, Holmes B. Hemangioendothelioma of the cavernous sinus: Case report. Neurosurgery. 1998. 42: 1153-5
71. Puca A, Meglio M, Rollo M, Zannoni GF. Intracranial epithelioid hemangioendothelioma: Case report. Neurosurgery. 1996. 38: 399-401
72. Raheja A, Suri A, Singh S, Kumar R, Kumar R, Nambirajan A. Multimodality management of a giant skull base hemangioendothelioma of the sphenopetroclival region. J Clin Neurosci. 2015. 22: 1495-8
73. Rath S, Mohanty B, Syasamal BC. Cerebral hemangioendothelioma. J Indian Med Assoc. 1970. 54: 372-3
74. Reis-Filho JS, Paiva ME, Lopes JM. Congenital composite hemangioendothelioma: Case report and reappraisal of the hemangioendothelioma spectrum. J Cutan Pathol. 2002. 29: 226-31
75. Requena L, Luis Díaz J, Manzarbeitia F, Carrillo R, Fernández-Herrera J, Kutzner H. Cutaneous composite hemangioendothelioma with satellitosis and lymph node metastases. J Cutan Pathol. 2008. 35: 225-30
76. Rocha Oliveira PC, Alcantara FP, Souza-Vianna PE, Brito AP. Cerebral epithelioid hemangioendothelioma with thoracic simultaneous involvement: Advanced MRI features. Arq Neuropsiqtr. 2012. 70: 637-8
77. Rokni GR, Montazer F, Sharifian M, Goldust M. Composite hemangioendothelioma of the forehead and right eye; a case report. BMC Dermatol. 2017. 17: 15
78. Rumana M, Khursheed N, Ramzan A. Congenital occipital encephalocele with Dabska tumor: Report of an unusual case. Pediatr Neurosurg. 2012. 48: 48-50
79. Rushing EJ, White JA, D’Alise MD, Chason DP, White CL, Bigio EH. Primary epithelioid hemangioendothelioma of the clivus. Clin Neuropathol. 1998. 17: 110-4
80. Sapunar J, Roa JC, Moscoso S. Hipofosfatemia revertida al extirpar hemangioendotelioma compuesto del dedo mayor del pie [Reversion of hypophosphatemia after the excision of a composite hemangioendothelioma in the great toe]. Rev Med Chil. 2003. 131: 909-14
81. Stojsic Z, Brasanac D, Stojanovic M, Boricic M. Cutaneous composite hemangioendothelioma: Case report and review of published reports. Ann Saudi Med. 2014. 34: 182-8
82. Süß P, Volz F, Lang C, Staszewksi O, Palmedo G, Taschner CA. A case of large meningeal epithelioid hemangioendothelioma with WWTR1-CAMTA1 gene rearrangement and slow growth over 15 years. J Neuropathol Exp Neurol. 2018. 77: 871-6
83. Sumrall A, Fredericks R, Berthold A, Shumaker G. Lenalidomide stops progression of multifocal epithelioid hemangioendothelioma including intracranial disease. J Neurooncol. 2010. 97: 275-7
84. Tammam AG, Lewis PD, Crockard HA. Cerebello-pontine angle epithelioid hemangioendothelioma in a 4-year-old boy. Childs Nerv Syst. 1997. 13: 648-50
85. Tancredi A, Puca A, Carbone A. Multifocal cerebral hemangioendothelioma. Case report and review of the literature. Acta Neurochir. 2000. 142: 1157-61
86. Taratuto AL, Zurbriggen G, Sevlever G, Saccoliti M. Epithelioid hemangioendothelioma of the central nervous system immunohistochemical and ultrastructural observations of a pediatric case. Pediatr Neurosurg. 1988. 14: 11-4
87. Tateishi J, Saeki H, Ito K, Nakagawa H, Fukunaga M. Cutaneous composite hemangioendothelioma on the nose treated with electron beam. Int J Dermatol. 2013. 52: 1618-9
88. Tejera-Vaquerizo A, Herrera-Ceballos E, Bosch-García R, Fernandez-Orland A, Matilla A. Composite cutaneous hemangioendothelioma on the back. Am J Dermpathol. 2008. 30: 262-4
89. Tian WZ, Yu XR, Wang WW, Zhang BO, Xia JG, Liu HQ. Computed tomography and magnetic resonance features of intracranial hemangioendothelioma: A study of 7 cases. Oncol Lett. 2016. 11: 3105-10
90. Tronnier M, Vogelbruch M, Kutzner H. Spindle cell hemangioma and epithelioid hemangioendothelioma arising in an area of lymphedema. Am J Dermpathol. 2006. 28: 223-7
91. Tsai JW, Huang HY, Lee JC, Yen YS, Tung CL, Huang CC. Composite haemangioendothelioma: Report of four cases with emphasis on atypical clinical presentation. Pathology. 2011. 43: 176-80
92. Tsuchiya T, Oya S, Mori H, Matsui T. Multiple hemorrhagic intraparenchymal tumors presenting with fatal intracranial hypertension: A rare manifestation of systemic epithelioid hemangioendothelioma. Surg Neurol Int. 2015. 6: 156
93. Utas S, Canoz O, Ferahbas A, Ozcan N. Composite cutaneous haemangioendothelioma treated with interferon. J Eur Acad Dermatol Venereol. 2008. 22: 503-5
94. Venizelos ID, Paradinas FJ. Primary pediatric intracranial epithelioid hemangioendothelioma. Histopathology. 2002. 41: 172-4
95. Watanabe T, Saito N, Shimaguchi H, Fujimaki H, Kamiya M, Nakazato Y. Primary epithelioid hemangioendothelioma originating in the lower petroclival region: case report. Surg Neurol. 2003. 59: 429-33
96. WHO Classification of Tumours Editorial Boa, editors. Soft tissue and bone tumours. WHO classification of tumours series. Lyon, France: International Agency for Research on Cancer; 2020. 3: Available from: https://tumourclassification.iarc.who.int/chapters/33 [Last accessed on 2023 Dec 30]
97. Xie S, Wang X, Zhang Y, Cheng J. Intracranial pseudomyogenic hemangioendothelioma: A case report. Asian J Surg. 2023. 46: 6067-8
98. Yamamoto F, Yamagiwa H, Iwamoto F, Kasugai T. A case of primary intracranial epithelioid hemangioendothelioma. No Shinkei Geka. 2018. 46: 35-40
99. Yeo SK, Kim JH, Kim CJ, Lee JK. Intracranial epithelioid hemangioendothelioma. J Korean Neurosurg Soc. 2007. 42: 129-31
100. Yoda Y, Ohashi M. A case of composite hemangioendothelioma arising from the spleen. Jpn J Clin Oncol. 2012. 42: 770
101. Zhang J, Wang Y, Geng D. Intracranial epithelioid hemangioendothelioma: An unusual CTA finding in one case. Br J Neurosurg. 2010. 24: 294-5
102. Zhang J, Wu B, Zhou GQ, Zhang RS, Wei X, Yu B. Composite hemangioendothelioma arising from the kidney: Case report with review of the literature. Int J Clin Exp Path. 2013. 6: 1935-41
103. Zhang X, Wang S, Jin M. The first case of composite hemangioendothelioma in the heart. Heart Surg Forum. 2022. 25: E284-7
104. Zheng J, Liu L, Wang J, Wang S, Cao Y, Zhao J. Primary intracranial epithelioid hemangioendothelioma: A low-proliferation tumor exhibiting clinically malignant behavior. J Neurooncol. 2012. 110: 119-27
105. Zheng J, Li P, Ma S, Geng M. Epithelioid hemangioendothelioma of the meninges mimicking metastatic carcinoma: A case report. Clin Neuropathol. 2013. 32: 324-7