- Department of Neurosurgery, Saiseikai Fukuoka General Hospital, Fukuoka, Japan
- Department of Neurology, Saiseikai Fukuoka General Hospital, Fukuoka, Japan
- Department of Neurosurgery, Kurume University School of Medicine, Kurume, Japan.
Correspondence Address:
Yukihiko Nakamura, Department of Neurosurgery, Saiseikai Fukuoka General Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_517_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yukihiko Nakamura1, Chihiro Takashima1, Takahisa Nonaka1, Taku Ohkubo1, Takayuki Kawano1, Akira Okura1, Daisuke Kondou2, Kazutaka Sonoda2, Masaru Hirohata3, Motohiro Morioka3. Early recanalization and vasospasm after endovascular treatment in a case of ruptured vertebral artery dissecting aneurysm associated with COVID-19. 08-Sep-2023;14:324
How to cite this URL: Yukihiko Nakamura1, Chihiro Takashima1, Takahisa Nonaka1, Taku Ohkubo1, Takayuki Kawano1, Akira Okura1, Daisuke Kondou2, Kazutaka Sonoda2, Masaru Hirohata3, Motohiro Morioka3. Early recanalization and vasospasm after endovascular treatment in a case of ruptured vertebral artery dissecting aneurysm associated with COVID-19. 08-Sep-2023;14:324. Available from: https://surgicalneurologyint.com/surgicalint-articles/12531/
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic has caused significant structural changes in acute care hospitals. COVID-19-associated stroke has gained attention, with abnormal coagulation and vascular endothelial damage being recognized. While ischemic cases are commonly reported, hemorrhagic cases have also been reported. This report presents a case of ruptured vertebral artery dissection aneurysm associated with COVID-19, resulting in subarachnoid hemorrhage (SAH). The treatment course, challenges in managing cerebral vasospasm, and early recanalization achieved through endovascular therapy are described.
Case Description: A 67-year-old male patient was brought to our hospital for emergency treatment of impaired consciousness that occurred while recovering from COVID-19. He underwent endovascular internal trapping using coils, and although the rupture did not recur, he required long-term tracheal management, which resulted in a cerebral infarction caused by cerebral vasospasm. In addition, early recanalization was seen, which required retreatment.
Conclusion: This case highlights the challenges in managing COVID-19-associated SAH and emphasizes the need for infection control measures and proper postoperative care. Establishing protocols for detecting and managing cerebral vasospasm is essential.
Keywords: Coronavirus disease 2019, Delayed cerebral infarction, Endovascular internal trapping, Vasospasm, Vertebral artery dissecting aneurysm
INTRODUCTION
The unprecedented COVID-19 (coronavirus disease 2019) pandemic has brought significant changes to the structure of acute care hospitals. COVID-19-associated stroke has gained attention in recent years.[
CASE DESCRIPTION
A 67-year-old male, cab driver. Past History: None noted.
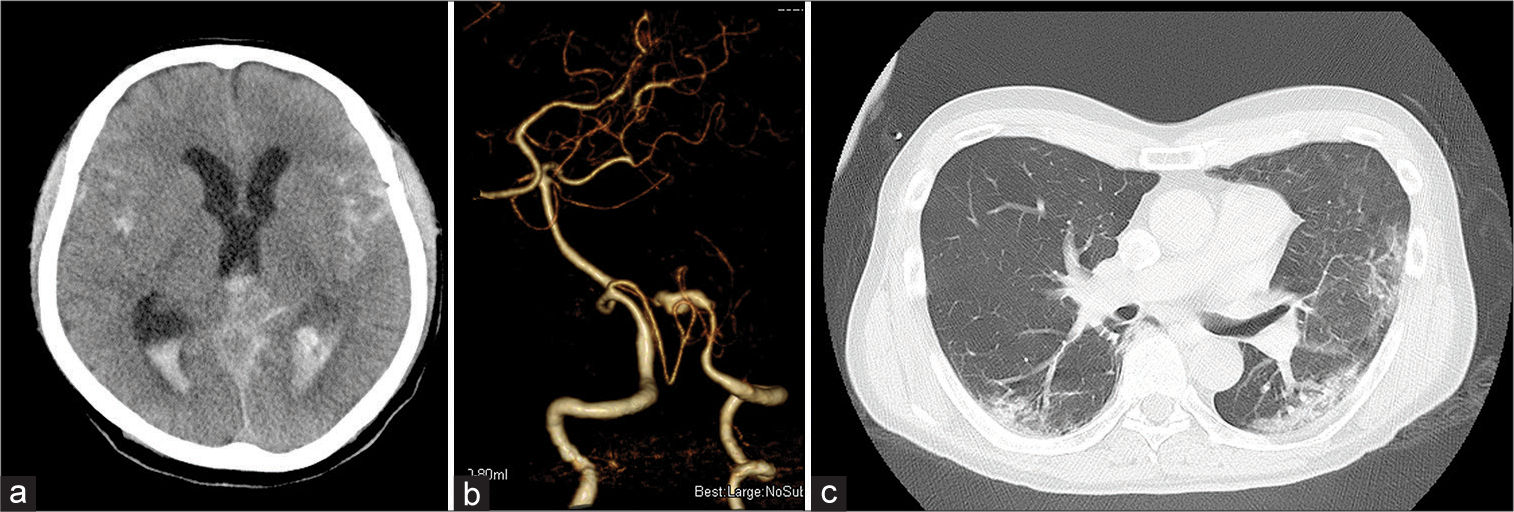
On the 3rd day of COVID-19 onset, he did not answer his family’s phone calls, and when the hotel staff checked on him, they found that he had collapsed in his room and was rushed to the hospital. Neurological findings: Glasgow Coma Score: 9 (E2V3M4) with no apparent paralysis. Imaging findings: Computed tomography (CT) scanning revealed SAH, intraventricular hemorrhage, and hydrocephalus [
Figure 1:
Computed tomography (CT) and three-dimensional CT angiogram on admission after COVID-19 onset day 3 (a) diffuse subarachnoid hemorrhage with intraventricular hemorrhage and hydrocephalus. (b) Pearls and strings are seen in the left vertebral artery. (c) Chest CT shows multiple frosted shadows are seen in the bilateral lung fields.
Treatment plan
The diagnosis of SAH (Hunt and Kosnik: grade 4, world federation of neurosurgical societies (WFNS): grade 4) due to a ruptured vertebral artery dissecting aneurysm was determined to be the PICA distal-type, and the development of a contralateral VA was sufficient flow and endovascular internal trapping was planned.
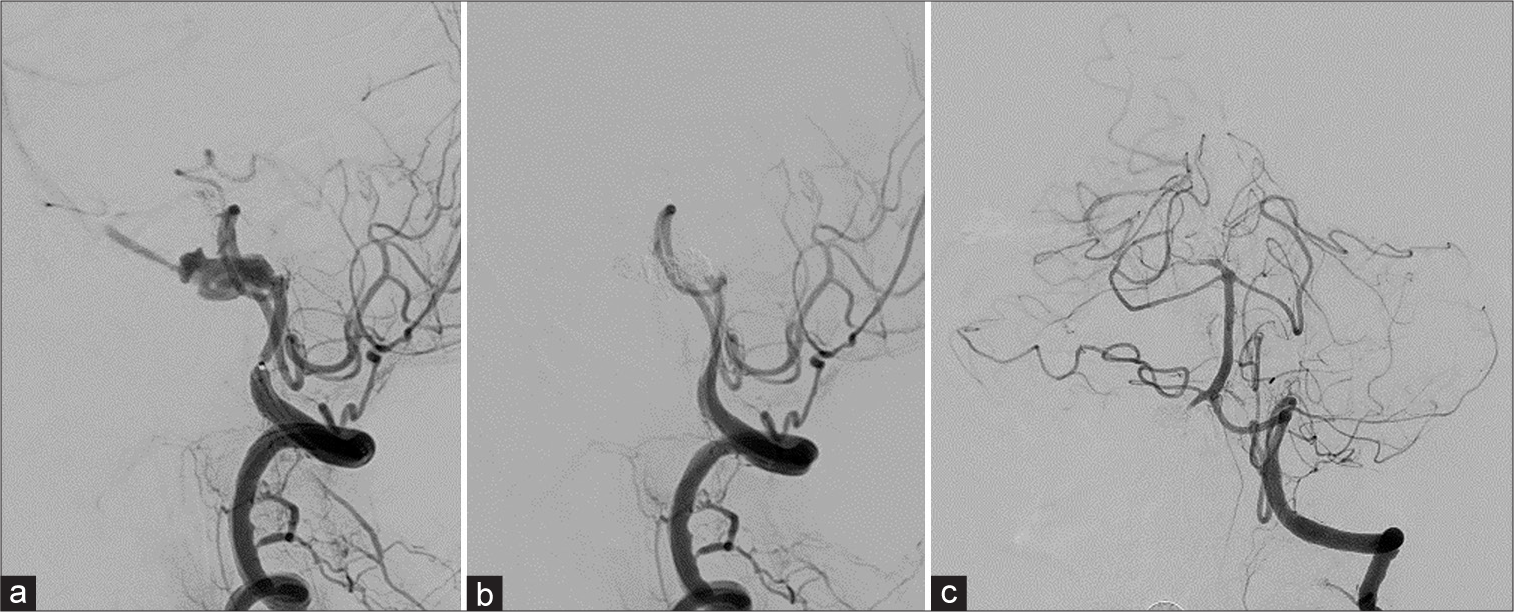
Endovascular treatment was performed with the minimum required number of surgeons and medical staff under full personal protective equipment (full-PPE) in the angio (ANGIO) suite with a negative-pressure ventilation system. Under general anesthesia, both femoral arteries were punctured and a 5Fr Guiding sheath was placed in the right VA, and a 4Fr catheter was placed in the left VA to control digital subtraction angiography (DSA). DSA revealed that the aneurysm was PICA distal-type with a dome measuring 12.6 mm × 6 mm [
Figure 2:
Digital subtraction angiography of the right vertebral artery (VA) (a and b) and left VA (c) (a) right vertebral artery dissecting aneurysm (VAG), lateral view. The aneurysm was present just after the posterior inferior cerebellar artery (PICA) branch. (b) Right VAG, lateral view. Flow arrest was seen in the right VA while the PICA was preserved. (c) Left VAG, frontal view. After internal trapping, the left VA was perfused sufficiently, retrograde flow to the right VA, and a branch of the anterior spinal artery was observed.
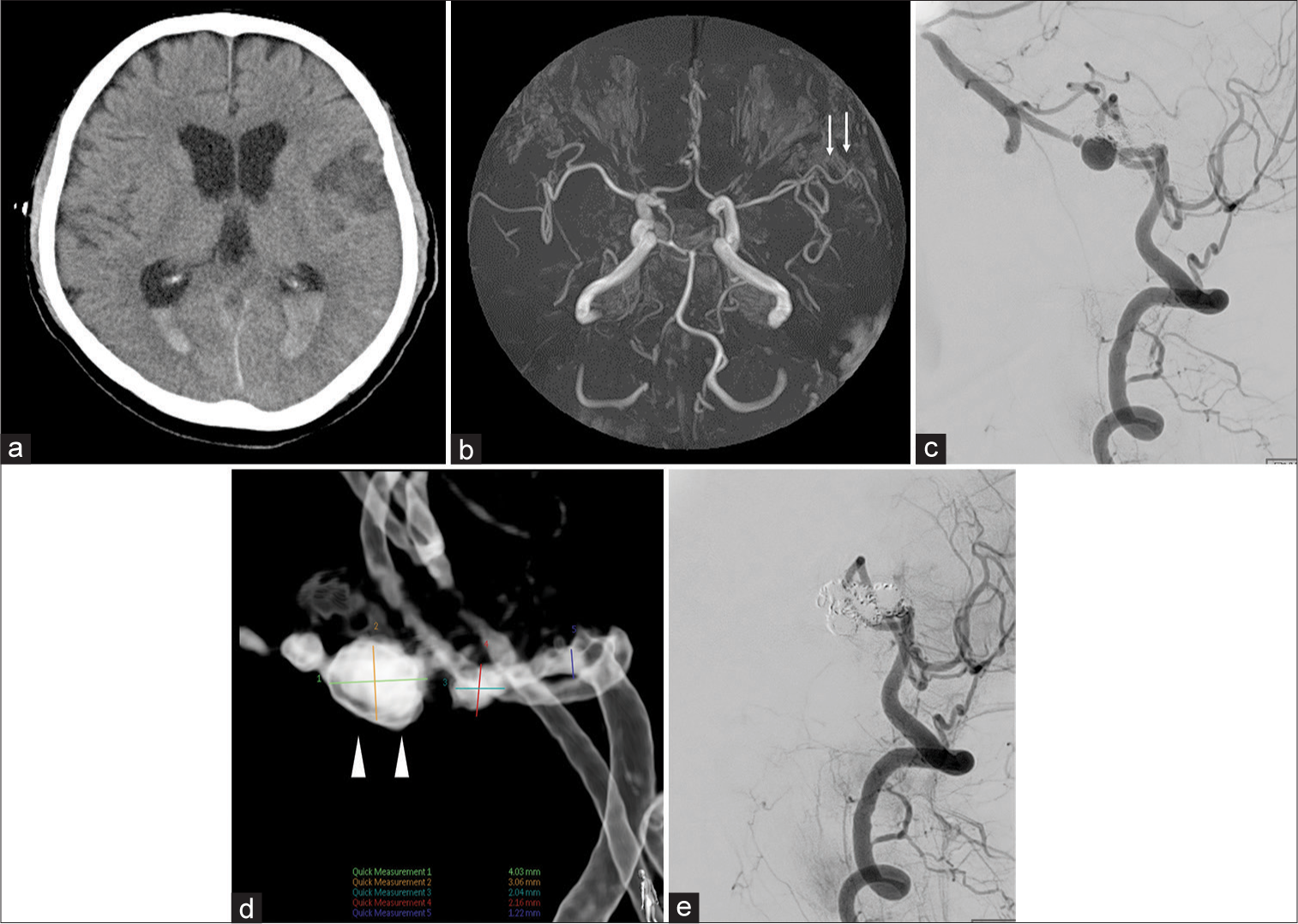
Postoperatively, the patient was managed as in the case of a normal severe SAH, including strict fluid management, blood pressure maintenance, and the administration of fasudil hydrochloride and sodium ozagrel natrium (nimodipine is not applicable in our country). The only deviation from regular treatment, considering the risk of secondary infection due to extubation, is that we continued to manage the patient by intubation. We performed neurological examinations and found no paralysis or sensory disturbance every few hours. Furthermore, the patients have undergone transcranial Doppler (TCD) examinations every day, which revealed results that were not significantly elevated (mean flow velocity was 50–80 cm/s). The patient was extubated 10 days after hospitalization, following two consecutive negative COVID – polymerase chain reaction (PCR) results. At that time, motor aphasia was observed, and CT revealed a focal cerebral infarction in the left frontal lobe [
Figure 3:
Computed tomography (a) and magnetic resonance angiography (MRA) (b) on the 10 days after hospitalization. Digital subtraction angiography of the right vertebral artery (VA) on the 30 days after hospitalization (c-e). (a) Part of the left frontal lobe showed a low-intensity zone. (b) MRA showing mild stenosis of the left distal middle cerebral artery (white arrow). (c) Right vertebral angiography (VAG), lateral view. Recanalization is seen in the right VA. (d) Right VAG, lateral view. An aneurysm-like dilation (white arrowhead) is seen at the lower part of the coil mass. (e) Right VAG, lateral view. After re-embolized, flow arrest was seen in the right VA while the posterior inferior cerebellar artery (PICA) was preserved.
A ventriculoperitoneal shunt was also performed for the complication of hydrocephalus. The patient was transferred to a different hospital for rehabilitation on the 50th day, and his symptoms gradually recovered, with mild motor aphasia persisting at 1 year after onset and the modified Rankin Scale 1.
DISCUSSION
The association between COVID-19 and stroke has attracted attention in recent times.[
The risk of developing SAH is currently unknown and considered controversial;[
Otherwise, to consider the importance of the management of COVID-19-associated SAH, a report on the management of aneurysmal SAH under COVID-19 prevalence has been published.[
In the present case, the patient developed recurrence after internal trapping and similar cases of recurrence have been reported in the past.[
During the acute phase in the COVID-19-associated vasculopathies, infection by SARS-CoV-2 seems to have specific direct and indirect effects on the endothelium, immune, and coagulation systems, thus promoting endothelial dysfunction, immunothrombosis, and formation of neutrophil extracellular traps.[
CONCLUSION
We experienced a case of a ruptured VA dissecting aneurysm associated with COVID-19. Thorough infection control measures are essential from the initial stages to the postoperative term. Postoperative management alone may be insufficient. It is necessary to consider the establishment of a preventive and examination system for cerebral vasospasm and keep in mind the possibility of recurrence.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bond KM, Krings T, Lanzino G, Brinjikji W. Intracranial dissections: A pictorial review of pathophysiology, imaging features, and natural history. J Neuroradiol. 2021. 48: 176-88
2. Dodd WS, Jabbour PM, Sweid A, Tjoumakaris S, Gooch MR, Al Saiegh F. Aneurysmal subarachnoid hemorrhage in patients with coronavirus disease 2019 (COVID-19): A case series. World Neurosurg. 2021. 153: e259-64
3. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A. Neurological associations of COVID-19. Lancet Neurol. 2020. 19: 767-83
4. Greistorfer T, Jud P. Pathophysiological aspects of COVID-19-associated vasculopathic diseases. Thromb Haemost. 2023. p.
5. Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020. 11: 322-5
6. Humayun L, Smith C, Li W, Zhang YS, Park C, Feng W. SARS-CoV-2-related vascular injury: Mechanisms, imaging and models. Microphysiol Syst. 2021. 5: 1
7. Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected code stroke: Hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020. 51: 1891-5
8. Kikuchi Y, Sugiu K, Tokunaga K, Nishida A, Nishimura T, Date I. Case of a ruptured vertebral artery dissecting aneurysm recanalized after internal trapping. No Shinkei Geka. 2007. 35: 813-9
9. Mastantuono JM, Combescure C, Elia N, Tramèr MR, Lysakowski C. Transcranial Doppler in the diagnosis of cerebral vasospasm: An updated meta-analysis. Crit Care Med. 2018. 46: 1665-72
10. Morassi M, Bigni B, Cobelli M, Giudice L, Bnà C, Vogrig A. Bilateral carotid artery dissection in a SARS-CoV-2 infected patient: Causality or coincidence?. J Neurol. 2020. 267: 2812-4
11. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke. 2021. 16: 137-49
12. Nguyen TN, Haussen DC, Qureshi MM, Yamagami H, Fujinaka T, Mansour OY. Decline in subarachnoid haemorrhage volumes associated with the first wave of the COVID-19 pandemic. Stroke Vasc Neurol. 2021. 6: 542-52
13. Nguyen TN, Jadhav AP, Dasenbrock HH, Nogueira RG, Abdalkader M, Ma A. Subarachnoid hemorrhage guidance in the era of the COVID-19 pandemic-An opinion to mitigate exposure and conserve personal protective equipment. J Stroke Cerebrovasc Dis. 2020. 29: 105010
14. Ravindra VM, Grandhi R, Delic A, Hohmann S, Shippey E, Tirschwell D. Impact of COVID-19 on the hospitalization, treatment, and outcomes of intracerebral and subarachnoid hemorrhage in the United States. PLoS One. 2021. 16: e0248728
15. Sadahiro H, Shirao S, Yoneda H, Ishihara H, Oku T, Inamura A. Decreased flow velocity with transcranial color-coded duplex sonography correlates with delayed cerebral ischemia due to peripheral vasospasm of the middle cerebral artery. J Stroke Cerebrovasc Dis. 2016. 25: 2352-9
16. Sato T, Miura Y, Yasuda R, Toma N, Suzuki H. Vertebral artery dissecting aneurysm rupture under severe COVID-19. Brain Hemorrhages. 2022. 3: 210-3
17. Sawada M, Kaku Y, Yoshimura S, Kawaguchi M, Matsuhisa T, Hirata T. Antegrade recanalization of a completely embolized vertebral artery after endovascular treatment of a ruptured intracranial dissecting aneurysm. Report of two cases. J Neurosurg. 2005. 102: 161-6
18. Yamada T, Kato T, Ishiguro M, Shirakami S, Imai S. Stent placement for ischemic intracranial carotid artery dissection in a patient with COVID-19. No Kekkannai Chiryo (NKC). 2022. 7: 13-9
19. You Y, Niu Y, Sun F, Zhang J, Huang S, Ding P. Impact of COVID-19 pandemic on haemorrhagic stroke admissions: A systematic review and meta-analysis. BMJ Open. 2021. 11: e050559