- Department of Medicine, Demerdash Hospital, Cairo, Egypt.
- Department of Neurosurgery, Al-Ahly Bank Hospital, Nasr City, Egypt.
- Department of Medicine, Zagazig University Hospitals, Zagazig, Egypt.
- Department of Surgery, Demerdash Hospital, Cairo, Egypt.
- Department of Medicine, Fayoum University, Fayoum, Egypt.
Correspondence Address:
Hieder Al-Shami
Department of Neurosurgery, Al-Ahly Bank Hospital, Nasr City, Egypt.
DOI:10.25259/SNI_132_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Diab1, Hieder Al-Shami2, Ahmed Negida3, Ahmed Gadallah4, Hossam Farag3, Doaa Mahmoud Elkadi5, Mo’tasem Muhamed Gaber4, Mahmoud Ahmed Ebada3. Efficacy and safety of polyethylene glycol dural sealant system in cranial and spinal neurosurgical procedures: Meta-analysis. 26-Apr-2021;12:182
How to cite this URL: Ahmed Diab1, Hieder Al-Shami2, Ahmed Negida3, Ahmed Gadallah4, Hossam Farag3, Doaa Mahmoud Elkadi5, Mo’tasem Muhamed Gaber4, Mahmoud Ahmed Ebada3. Efficacy and safety of polyethylene glycol dural sealant system in cranial and spinal neurosurgical procedures: Meta-analysis. 26-Apr-2021;12:182. Available from: https://surgicalneurologyint.com/surgicalint-articles/10763/
Abstract
Background: We aimed to assess the efficacy of polyethylene glycol (PEG) dura sealant to achieve watertight closure, prevention of cerebrospinal fluid (CSF) leak and to investigate its possible side effects.
Methods: We searched Medline (through PubMed), Scopus, and the Cochrane Library through December 2019. We included articles demonstrating cranial or spinal procedures with the use of PEG material as a dural sealant. Data on intraoperative watertight closure, CSF leak, and surgical complications were extracted and pooled in a meta-analysis model using RevMan version 5.3 and OpenMeta (Analyst).
Results: Pooling the controlled trials showed that PEG resulted in significantly more intraoperative watertight closures than standard care (risk ratio [RR] = 1.44, 95% confidence interval [CI] [1.24, 1.66]). However, the combined effect estimate did not reveal any significant difference between both groups in terms of CSF leaks, the incidence of surgical site infections, and neurological deficits (P = 0.7, 0.45, and 0.92, respectively). On the other hand, pooling both controlled and noncontrolled trials showed significance in terms of leak and neurological complications (RR = 0.0238, 95% CI [0.0102, 0.0373] and RR = 0.035, 95% CI [0.018, 0.052]). Regarding intraoperative watertight closure, the overall effect estimate showed no significant results (RR=0.994, 95% CI [0.986, 1.002]).
Conclusion: Dura seal material is an acceptable adjuvant for dural closure when the integrity of the dura is questionable. However, marketing it as a factor for the prevention of surgical site infection is not scientifically proved. We suggest that, for neurosurgeons, using the dural sealants are highly recommended for duraplasty, skull base approaches, and in keyhole approaches.
Keywords: Dura sealant, Dural closure, Hydrogel, Polyethylene glycol
INTRODUCTION
Cerebrospinal fluid (CSF) leakage after neurosurgical operations is a common phenomenon at either infratentorial or supratentorial surgeries. It ranges from 10 to 25%.[
The main method of dural closure is the primary surgical closure; however, this is not enough to achieve a watertight approximation.[
At present, the techniques used to achieve a watertight dural closure include the application of interrupted sutures, dural replacement materials (duraplasty), and hemostatic agents.[
Polyethylene glycol (PEG)-based hydrogel is a new sealant used as an adjuvant to augment primary dural closure after craniotomy.[
In this meta-analysis of clinical trials, we aimed to assess the efficacy of PEG to achieve watertight closure of the dura and prevention of CSF leak and to investigate its possible side effects.
MATERIAL’S AND METHODS
We performed all steps of this systematic review in strict accordance with the Cochrane handbook of systematic reviews and meta-analysis. We also followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement guidelines while drafting our manuscript.[
Literature search strategy
We searched Medline (through PubMed), Scopus, and the Cochrane Library through December 2019, using the following keywords: “PEG,” “Hydrogel,” “Dural Sealant,” “Dural Closure,” “Neurosurgery,” “Cranial,” and “Spinal.”
No restrictions by language, country, or publication date were employed. We also searched the bibliography of eligible studies for relevant articles.
Eligibility criteria
We included both randomized controlled trials and noncontrolled studies assessing the use of PEG hydrogel for dura matter closure in cranial or spinal neurosurgical procedures.
We excluded nonhuman studies, studies from which data cannot be reliably extracted, duplicate references, case reports, and conference abstracts.
Selection of studies
We independently applied the selection criteria; eligibility screening was conducted in two steps, (a) titles and abstracts screening for matching the inclusion criteria and (b) full-text screening for eligibility to meta-analysis. Disagreements were resolved on discussion.
Outcomes of interest
We included studies reported at least one of the following outcomes: (1) intraoperative watertight closure, (2) CSF leak, and (3) surgical complications such as meningocele, surgical site infection, sepsis, subarachnoid hemorrhage, and pneumocephalus.
Data extraction
We independently extracted and tabulated data on the first author, publication year, study design, baseline characteristics of the study population, type of intervention including the type of prosthesis, study period, follow-up period, and relevant outcomes data. Disagreements were resolved on discussion.
Risk of bias (ROB) assessment
Two independent reviewers used the Cochrane ROB assessment tool, clearly described in Chapter 8.5 of the Cochrane handbook of systematic reviews of interventions 5.1.0. The Cochrane ROB assessment tool is designed to detect five types of bias, including selection bias (sequence generation and allocation concealment), performance bias (blinding of participants and investigators), detection bias (blinding of outcome assessors), attrition bias (incomplete outcome data), and reporting bias (selective outcome reporting).[
Data analysis
We included both controlled and noncontrolled studies. We used OpenMeta (Analyst) software to calculate an overall estimate and 95% confidence interval (CI) for the outcome in the experimental groups in both sets of studies. We used Review Manager software (version 5.3) to calculate the risk ratio (RR) and 95% CI for outcomes of the controlled studies.
Assessment of heterogeneity
Heterogeneity was assessed by visual inspection of the forest plots and measured by Q statistic and I2 statistic. Significant statistical heterogeneity was indicated by Q statistic P < 0.1 or by I2 more than 50%.
Publication bias
According to Egger’s et al., publication bias is not reliable for <10 pooled studies. Therefore, in the present study, we could not assess the existence of publication bias by Egger’s test for funnel plot asymmetry.
RESULTS
Literature search results
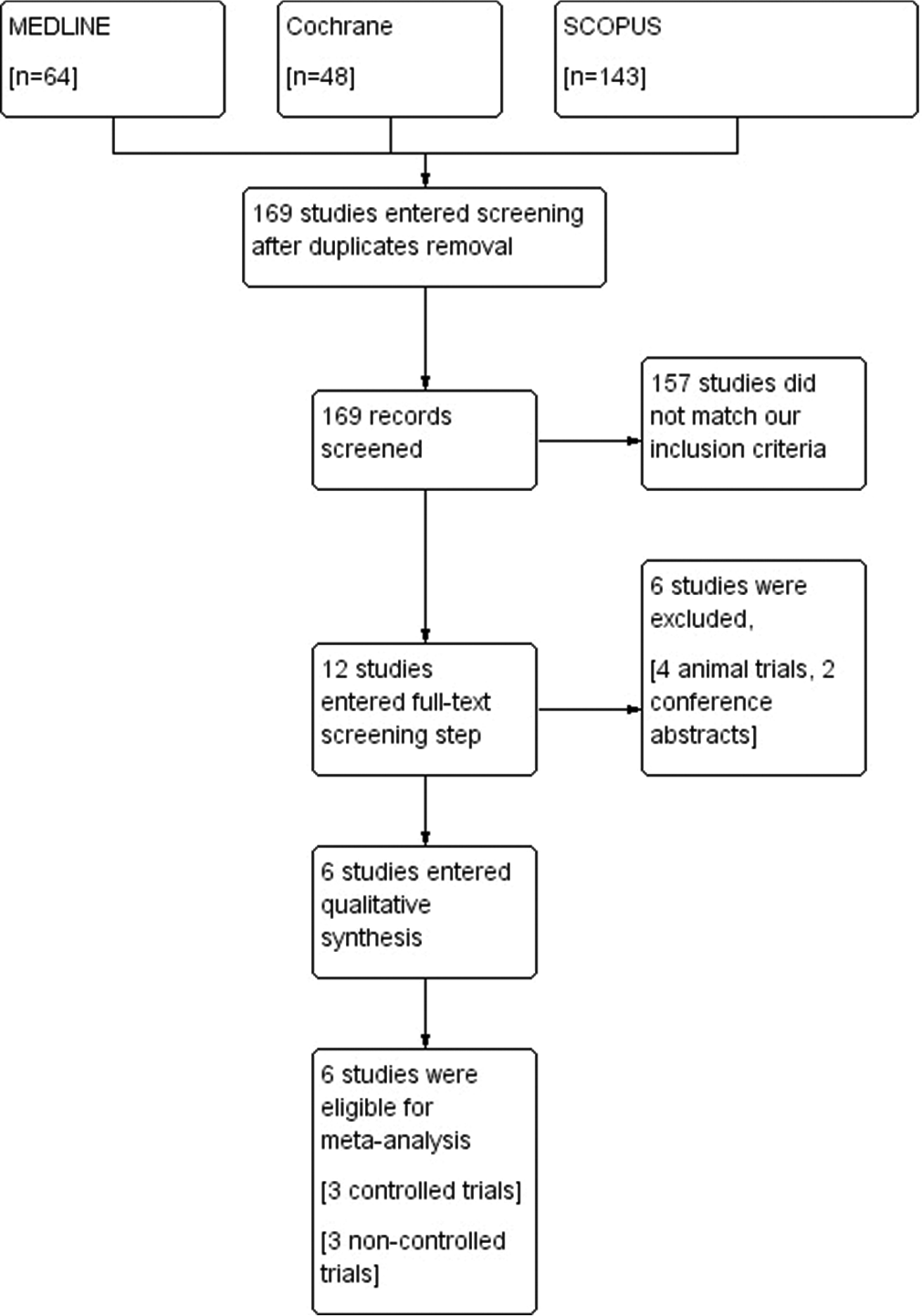
The results of searching databases yielded 255 studies. After excluding duplicates, 169 studies entered the screening phase. Twelve studies entered full-text screening, and a total of six studies were finally included in our study. Half of the included trials were controlled trials, and the other three studies were noncontrolled. [
Characteristics of the included studies’ population
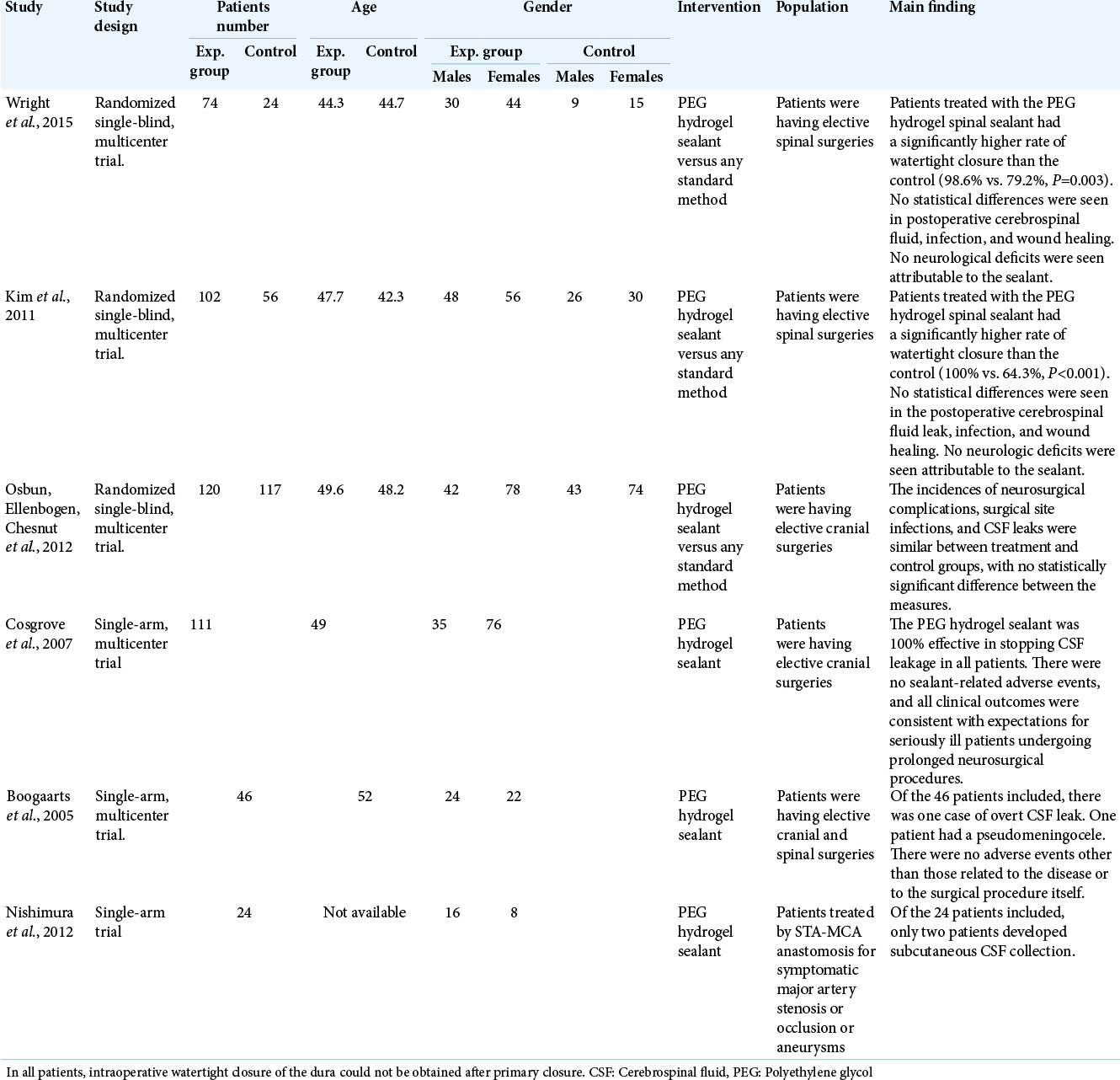
The included studies were six studies. Controlled and uncontrolled studies were in a ratio of 3:3. The total number of recruited patients from randomized studies was 493 patients. The uncontrolled studies contain 181 patients. The controlled trials measured PEG efficacy against conventional method used for dural closure. The summary of the baseline characteristics of the included studies is shown in [
Assessment of study validity
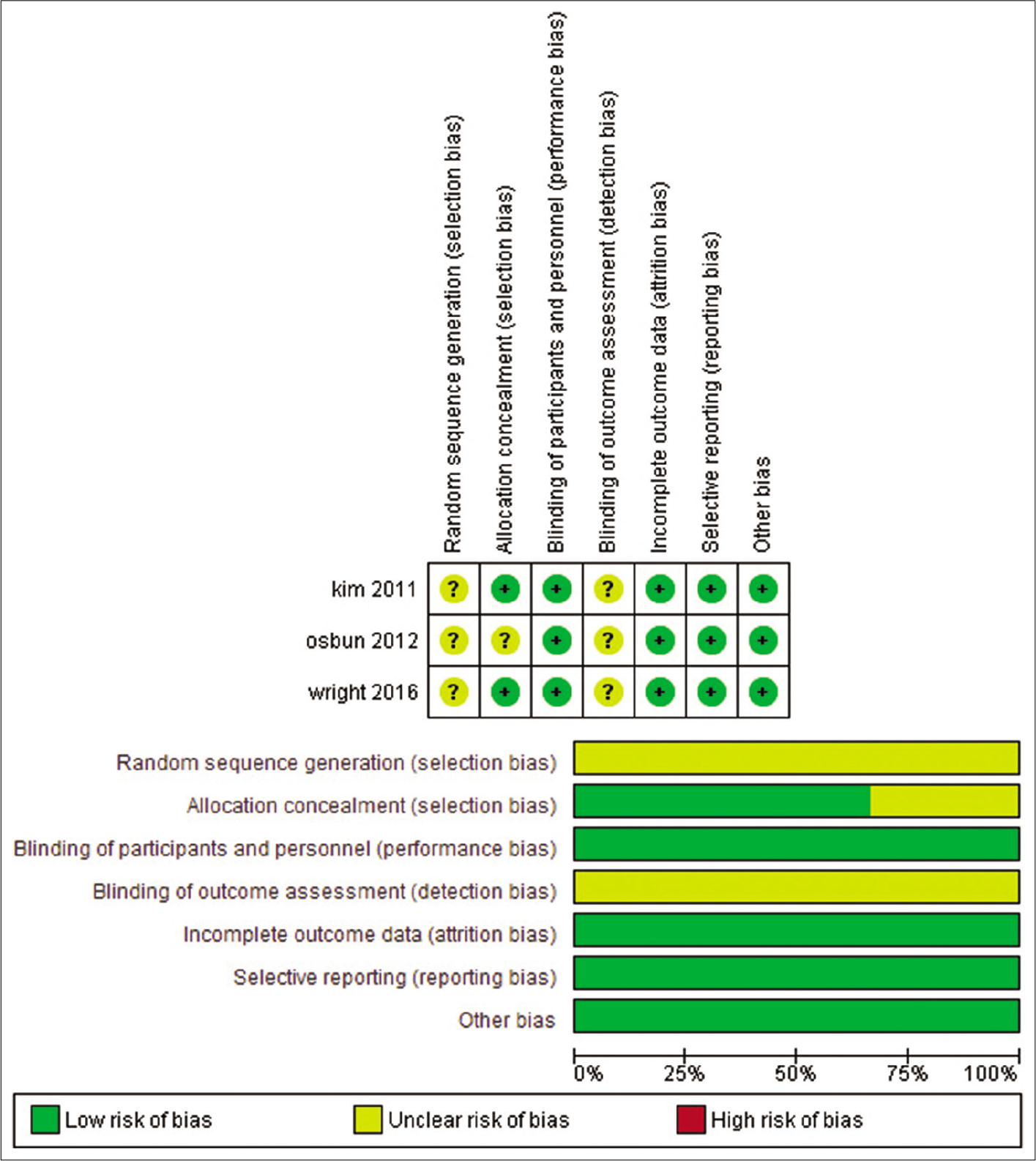
We detected an overall moderate ROB for the included clinical trials. Regarding randomization and blinding of outcome assessors, three studies did not report the methodology of randomization nor blinding of outcome assessors, therefore, were categorized as unclear ROB. As for allocation concealment, blinding of patients, attrition bias, and selective reporting, all studies were put to low risk, as they provided sufficient data for supporting these domains. Except for Osbun et al.,[
Synthesis of results
We conducted two analyses for selected outcomes; one for controlled trials and another single-arm analysis for both controlled and noncontrolled studies.
Analysis of controlled trials [ Figure 3 ]
Intraoperative watertight closure
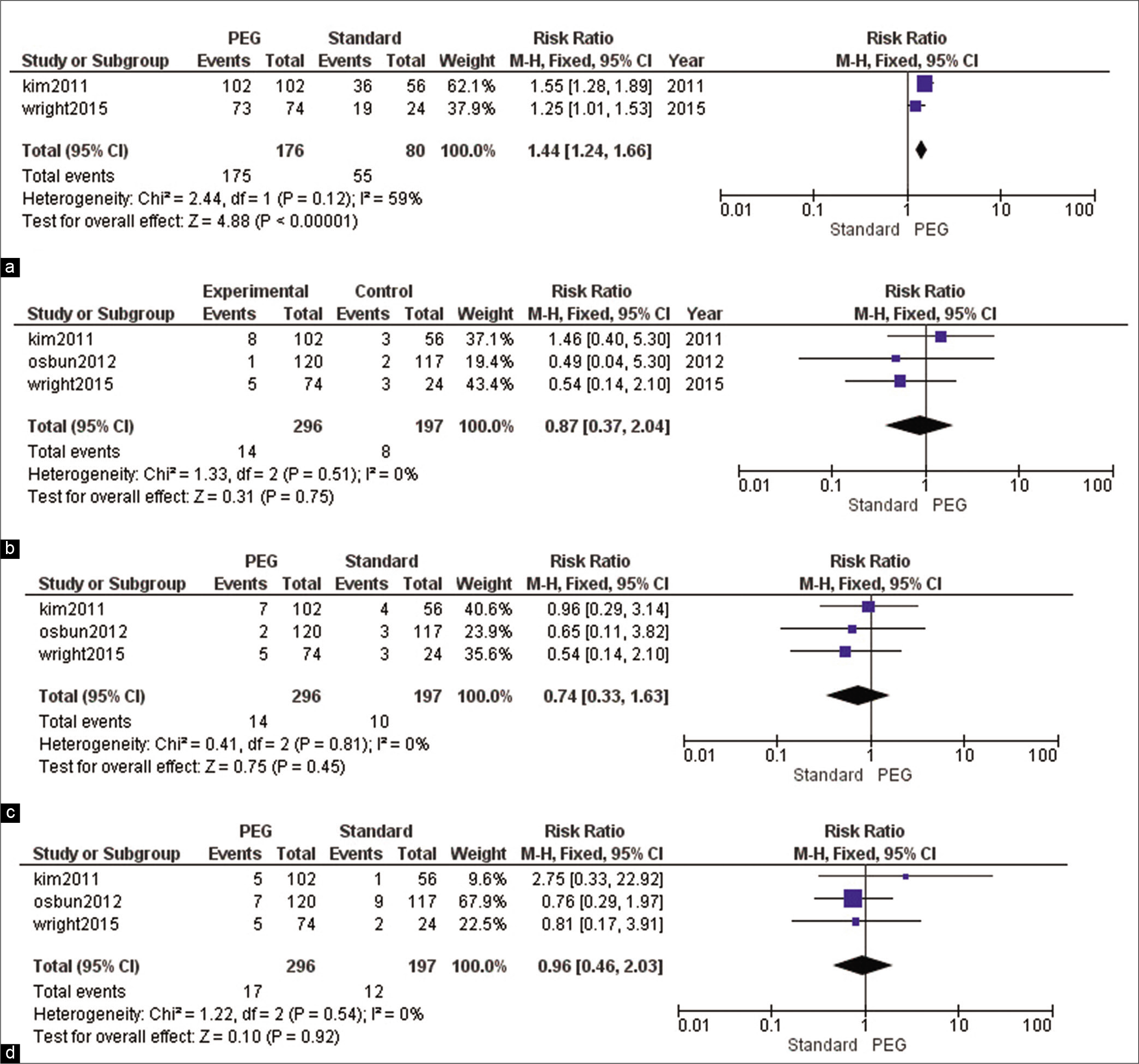
The overall RR showed that PEG resulted in significantly more intraoperative watertight closures than standard care (RR = 1.44, 95% CI [1.24, 1.66], P < 0.001). Pooled results were homogeneous (I2 = 59%, P = 0.12),
CSF leak
The combined effect estimate did not reveal any significant difference between both groups in terms of CSF leaks (RR = 0.87, 95% CI [0.37, 2.04], P = 0.7). Pooled results were homogenous (I2 = 0%, P = 0.5),
Surgical site infections
The net result of analysis did not favor any of the two groups regarding the incidence of surgical site infections (RR = 0.74, 95% CI [0.33, 1.63], P = 0.45). Pooled results were homogeneous (I2 = 0%, P = 0.8),
Neurological deficits
The combined RR did not show a significant difference between PEG and standard care (RR = 0.96, 95% CI [0.46, 2.03], P = 0.92). Pooled results were homogenous (I2 = 0%, P = 0.5),
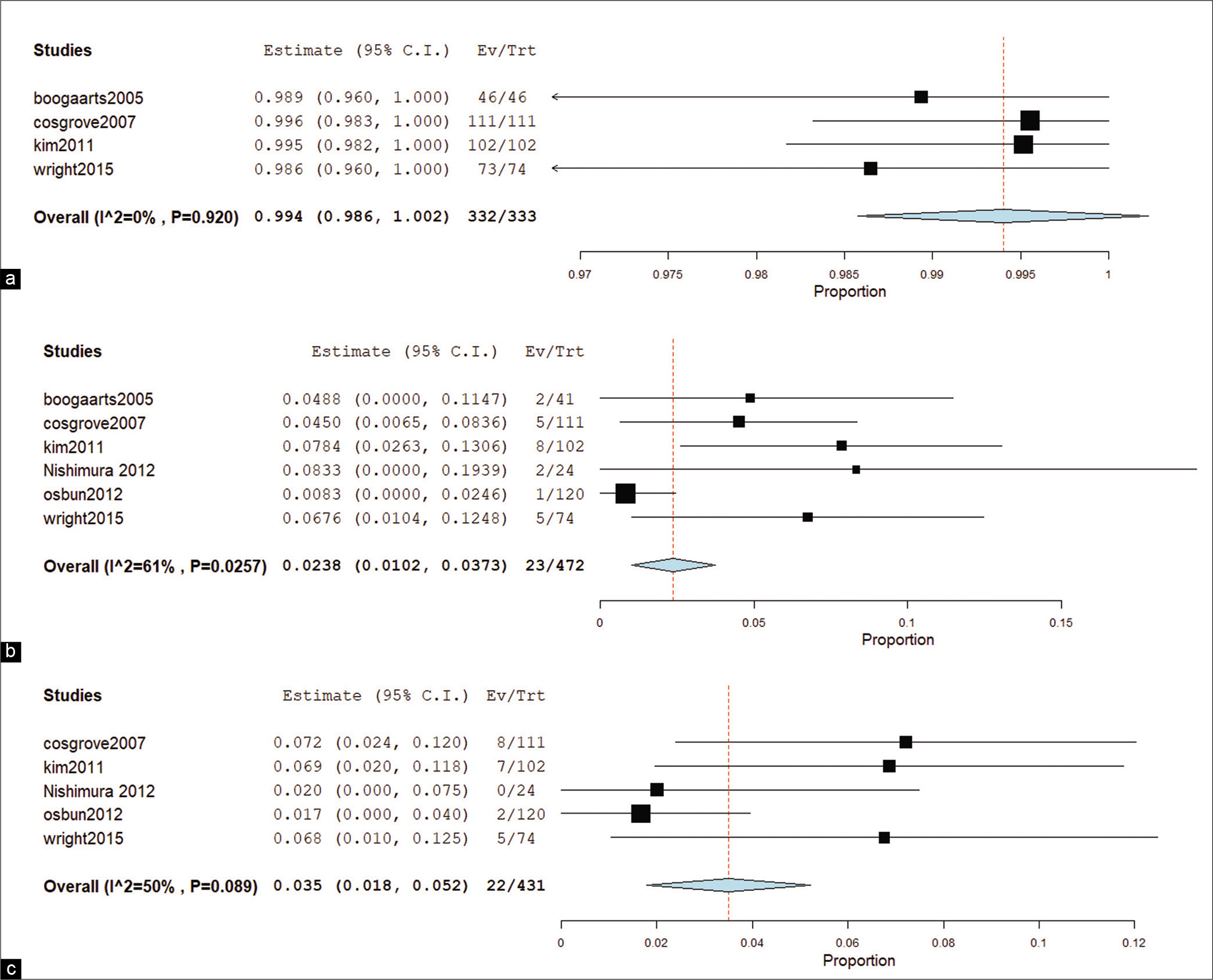
Analysis for both controlled and noncontrolled trials [ Figure 4 ]
Intraoperative watertight closure
The overall effect estimate did not reveal significant results (RR = 0.994, 95% CI [0.986, 1.002]). Pooled results were homogenous (I2 = 0%, P = 0.9),
CSF leak
Combined effect estimates and 95% CI showed marked significance (RR = 0.0238, 95% CI [0.0102, 0.0373]). Pooled results were heterogeneous (I2 = 61%, P = 0.026),
Neurological complications
The net results of neurological complications (pseudomeningocele, surgical site infection, and neurological deficit) from surgery revealed significant results (RR = 0.035, 95% CI [0.018, 0.052]). Pooled results were heterogeneous (I2 = 50%, P = 0.089),
DISCUSSION
Summary of main results
Analysis of controlled trials only revealed that PEG increases intraoperative watertight closures. However, it did not show any difference between PEG and standard care in terms of neurological deficits, postoperative CSF leaks, and surgical site infections.
Single-arm analysis of all trials showed no difference in terms of intraoperative watertight closures; this may be stronger evidence than controlled trials, as the strength of meta-analysis is increased by increasing the number of included studies, provided that results remain homogeneous. However, postoperative CSF leaks and surgical site infections were significant.
Significance of main results
Watertight closure is critical, and the dura seal is an excellent material to achieve zero leakage intraoperatively. However, stopping postoperative leakage cannot be guaranteed by the dura seal in controlled trials. Prevention of surgical site infection is the matter of achieving a good medium for healing and infection control rather than the application of foreign material.
Agreement and disagreement with the previous studies
Kim et al. studied the dural sealant in various procedures in neurosurgery; they found that using it was effective in the prevention of CSF fistula.[
They found that easy and effective sealing of the field was achieved by the dura seal.[
Osbun et al. conducted a randomized controlled trial used the same material in cranial surgeries in a group (n = 120) against the traditional method group (n = 117).[
In contrast, a different outcome was achieved by Green et al.[
Tew et al. conducted a large cohort study that included 17 centers to study the effectiveness of dural sealant versus traditional methods of dural closure in cranial surgeries.[
CONCLUSION AND RECOMMENDATIONS
Dura seal material is an acceptable adjuvant for dural closure when the integrity of the dura is in question. However, marketing it as a factor for the prevention of surgical site infection is not scientifically proved. We suggest that, for neurosurgeons, using the dural sealants are highly recommended for duraplasty, skull base approaches, and in keyhole approaches.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Cardona-Durán RF, Uribe JS, Baaj AA, Uribe J, Greenberg MS.editors. Spinal biologics. Handbook of Spine Surgery. New York: Thieme; 2016. p. 948
2. Beierlein W, Scheule AM, Antoniadis G, Braun C, Schosser R. An immediate, allergic skin reaction to aprotinin after reexposure to fibrin sealant. Transfusion. 2000. 40: 302-5
3. Boogaarts JD, Grotenhuis JA, Bartels RH, Beems T. Use of a novel absorbable hydrogel for augmentation of dural repair: Results of a preliminary clinical study. Neurosurgery. 2005. 57: 146-51
4. Wei SM, Pei MY, Pan WL, Thissen H, Tsai SW. Gelatin hydrogels reinforced by absorbable nanoparticles and fibrils cured in situ by visible light for tissue adhesive applications. Polymers. 2020. 12: 1113
5. Cosgrove GR, Delashaw JB, Grotenhuis JA, Tew JM, van Loveren H, Spetzler RF. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J Neurosurg. 2007. 106: 52-8
6. Ebada MA, Alkanj S, Ebada M, Abdelkarim AH, Diab A, Aziz MA. Safety and efficacy of levetiracetam for the management of levodopa-induced dyskinesia in patients with Parkinson’s disease: A systematic review. CNS Neurol Disord Drug Targets. 2019. 18: 317-25
7. Ebada MA, Elmatboly AM, Ali AS, Ibrahim AM, Fayed N, Faisal AF. An updated systematic review and meta-analysis about the safety and efficacy of infliximab biosimilar, CT-P13, for patients with inflammatory bowel disease. Int J Colorectal Dis. 2019. 34: 1633-52
8. Ebada MA, Fayed N, Fayed L, Alkanj S, Abdelkarim A, Farwati H. Efficacy of alpha-lipoic acid in the management of diabetes mellitus: A systematic review and meta-analysis. Iran J Pharm Res. 2019. 18: 2144-56
9. Eseonu CI, Goodwin CR, Zhou X, Theodros D, Bender MT, Mathios D. Reduced CSF leak in complete calvarial reconstructions of microvascular decompression craniectomies using calcium phosphate cement. J Neurosurg. 2015. 123: 1476-9
10. Gadallah AH, Ebada MA, Gadallah A, Ahmed H, Rashad W, Eid KA. Efficacy and safety of N-acetylcysteine as add-on therapy in the treatment of obsessive-compulsive disorder: A systematic review and meta-analysis. J Obsess Compuls Relat Disord. 2020. 25: 100529
11. George B, Matula C, Kihlström L, Ferrer E, Tetens V. Safety and efficacy of tachosil (absorbable fibrin sealant patch) compared with current practice for the prevention of cerebrospinal fluid leaks in patients undergoing skull base surgery: A randomized controlled trial. Neurosurgery. 2017. 80: 847-53
12. Green AL, Arnaud A, Batiller J, Eljamel S, Gauld J, Jones P. A multicentre, prospective, randomized, controlled study to evaluate the use of a fibrin sealant as an adjunct to sutured dural repair. Br J Neurosurg. 2015. 29: 11-7
13. Grotenhuis JA. Costs of postoperative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases. Surg Neurol. 2005. 64: 490-3
14. Higgins JP, Green S.editors. Cochrane Handbook for Systematic Reviews of Interventions-IdoStatistics. 2008. p.
15. Hutter G, von Felten S, Sailer MH, Schulz M, Mariani L. Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: A randomized controlled trial. J Neurosurg. 2014. 121: 735-44
16. Kanazawa R, Sato S, Iwamoto N, Teramoto A. Allergic reaction following arachnoid plasty with a fibrin sealant. Neurol Med Chir. 2010. 50: 608-10
17. Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (duraseal spinal sealant) as an adjunct to sutured dural repair in the Spine. Spine. 2011. 36: 1906-12
18. Messerer M, Cossu G, Daniel RT, Jouanneau E.editors. Letter to the editor: Knosp Grades 2-3 nonfunctioning pituitary adenomas. J Neurosurg. 2015. 122: 986-7
19. Nakamura H, Matsuyama Y, Yoshihara H, Sakai Y, Katayama Y, Nakashima S. The effect of autologous fibrin tissue adhesive on postoperative cerebrospinal fluid leak in spinal cord surgery: A randomized controlled trial. Spine. 2005. 30: E347-51
20. Nakayama N, Yano H, Egashira Y, Enomoto Y, Ohe N, Kanemura N. Efficacy, reliability, and safety of completely autologous fibrin glue in neurosurgical procedures: Single-center retrospective large-number case study. World Neurosurg. 2018. 109: e819-28
21. Nishimura K, Kimura T, Morita A. Watertight dural closure constructed with DuraSealTM for bypass surgery. Neurol Med Chir. 2012. 52: 521-4
22. Osbun JW, Ellenbogen RG, Chesnut RM, Chin LS, Connolly PJ, Cosgrove GR. A multicenter, single-blind, prospective randomized trial to evaluate the safety of a polyethylene glycol hydrogel (duraseal dural sealant system) as a dural sealant in cranial surgery. World Neurosurg. 2012. 78: 498-504
23. Patel MR, Caruso PA, Yousuf N, Rachlin J, Fibrin P. Glue therapy of cerebrospinal fluid. Am Roentgen Ray Soc. 2000. 175: 443-6
24. Pereira EA, Grandidge CA, Nowak VA, Cudlip SA. Cerebrospinal fluid leaks after transsphenoidal surgery effect of a polyethylene glycol hydrogel dural sealant. J Clin Neurosci. 2017. 44: 6-10
25. Preul MC, Bichard WD, Muench TR, Spetzler RF. Toward optimal tissue sealants for neurosurgery: Use of a novel hydrogel sealant in a canine durotomy repair model. Neurosurgery. 2003. 53: 1189-99
26. Shaffrey CI, Spotnitz WD, Shaffrey ME, Jane JA, Laws ER, Sekhar LN. Neurosurgical applications of fibrin glue: Augmentation of dural closure in 134 patients. Neurosurgery. 1990. 26: 207-10
27. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015. 350: g7647
28. Shimada Y, Hongo M, Miyakoshi N, Sugawara T, Kasukawa Y, Ando S. Dural substitute with polyglycolic acid mesh and fibrin glue for dural repair: Technical note and preliminary results. J Orthop Sci. 2006. 11: 454-8
29. Stoker M, Forbes J, Hanif R, Cooper C, Nian H, Konrad P. Decreased rate of CSF leakage associated with complete reconstruction of suboccipital cranial defects. J Neurol Surg Part B Skull Base. 2012. 73: 281-6
30. Takumi I, Mishina M, Hironaka K, Oyama K, Yamada A, Adachi K. Simple solution for preventing cerebrospinal fluid loss and brain shift during multitrack deep brain stimulation surgery in the semisupine position: Polyethylene glycol hydrogel dural sealant capping. Neurol Med Chir. 2013. 53: 1-6
31. Terasaka S, Taoka T, Kuroda S, Mikuni N, Nishi T, Nakase H. Efficacy and safety of non-suture dural closure using a novel dural substitute consisting of polyglycolic acid felt and fibrin glue to prevent cerebrospinal fluid leakage a non-controlled, open-label, multicenter clinical trial. J Mater Sci. 2017. 28: 69
32. Tew JM, Strong MJ, Alexanderwest G, Woo H, Couture DE, Wilson JA. A pivotal randomized clinical trial evaluating the safety and effectiveness of a novel hydrogel dural sealant as an adjunct to dural repair. Oper Neurosurg (Hagerstown). 2017. 13: 204-12
33. Allah AW, Yahya M, Elsaeidy KS, Alkanj S, Hamam K, ElSaady M. Clinical assessment of miRNA-23b as a prognostic factor for various carcinomas: A systematic review and meta-analysis. Meta Gene. 2020. 24: 100651
34. Wakamoto H, Miyazaki H, Orii M, Ishiyama N, Akiyama K, Konohana I. Aseptic meningitis as a complication caused by an allergic reaction after microvascular decompression: Two case reports. No Shinkei Geka. 2002. 30: 1331-5
35. Wright NM, Park J, Tew JM, Kim KD, Shaffrey ME, Cheng J. Spinal sealant system provides better intraoperative watertight closure than standard of care during spinal surgery: A prospective, multicenter, randomized controlled study. Spine. 2015. 40: 505-13
36. Yeom JS, Buchowski JM, Shen HX, Liu G, Bunmaprasert T, Riew KD. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: A retrospective matched pair analysis. Spine. 2008. 33: 543-7