Efficacy and safety of steroids for chronic subdural hematoma: A systematic review and meta-analysis
- Department of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan
- Department of Medicine, Shaheed Mohtarma Benazir Bhutto Medical College, Karachi, Pakistan
- Department of Medicine, Liaquat College of Medicine and Dentistry, Karachi, Pakistan
- Department of Medicine, Liaquat National Hospital and Medical College, Karachi, Pakistan,
- Department of Medicine, Ahfad University for Women, Omdurman, Sudan.
Correspondence Address:
Abdul Haseeb, Department of Medicine, Jinnah Sindh Medical University, Karachi, Pakistan.
DOI:10.25259/SNI_771_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdul Haseeb1, Muhammad Ashir Shafique1, Aashish kumar2, Moosa Abdur Raqib3, Zaib Un Nisa Mughal1, Rabia Nasir1, Syed Muhammad Sinaan Ali4, Tagwa Kalool Fadlalla Ahmad5, Muhammad Saqlain Mustafa1. Efficacy and safety of steroids for chronic subdural hematoma: A systematic review and meta-analysis. 29-Dec-2023;14:449
How to cite this URL: Abdul Haseeb1, Muhammad Ashir Shafique1, Aashish kumar2, Moosa Abdur Raqib3, Zaib Un Nisa Mughal1, Rabia Nasir1, Syed Muhammad Sinaan Ali4, Tagwa Kalool Fadlalla Ahmad5, Muhammad Saqlain Mustafa1. Efficacy and safety of steroids for chronic subdural hematoma: A systematic review and meta-analysis. 29-Dec-2023;14:449. Available from: https://surgicalneurologyint.com/surgicalint-articles/12690/
Abstract
Background: Chronic subdural hematoma (CSDH) is a condition characterized by the accumulation of fluid, blood, and blood breakdown products between the brain’s arachnoid and dura mater coverings. While steroids have been explored as a potential treatment option, their efficacy and safety remain uncertain. This meta-analysis and systematic review aimed to assess the impact of steroids on CSDH management, including mortality, recurrence, complications, and functional outcomes.
Methods: We conducted a comprehensive literature search in major electronic databases up to June 2023, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and Cochrane Handbook for Systematic Reviews and Interventions. Inclusion criteria encompassed adult patients with CSDH, the use of steroids as monotherapy or adjuvant therapy, and clearly defined outcomes. Randomized controlled trials and cohort studies meeting these criteria were included in the study.
Results: The initial search yielded 4315 articles, with 12 studies meeting the inclusion criteria. Our findings indicate a non-significant trend toward reduced mortality with steroids in combination with standard care (Odds ratios [OR] = 0.66, 95% confidence interval [CI] 0.20–2.18). However, substantial heterogeneity was observed (I2 = 70%). Sensitivity analysis, excluding influential studies, suggested a potential increased mortality risk associated with steroids (OR = 1.47, 95% CI 0.87–2.48). Steroids showed a possible benefit in reducing the recurrence of CSDH (OR = 0.58, 95% CI 0.20–1.67), but with significant heterogeneity (I2 = 89%). No clear advantage of steroids was observed in terms of functional outcomes at three months (modified Rankin scale scores). Furthermore, steroids were associated with a significantly higher incidence of adverse effects and complications (OR = 2.17, 95% CI 1.48–3.17).
Conclusion: Steroids may have a potential role in reducing CSDH recurrence but do not appear to confer significant advantages in terms of mortality or functional outcomes. However, their use is associated with a higher risk of adverse effects and complications. Given the limitations of existing studies, further research is needed to refine the role of steroids in CSDH management, considering patient-specific factors and treatment protocols.
Keywords: Chronic subdural hematoma, Complications, Dexamethasone, Meta-analysis, Mortality, Recurrence, Steroids, Systematic review
INTRODUCTION
Chronic subdural hematoma (CSDH) is an isolated collection of fluid, blood, and blood breakdown products placed on the surface of the brain between the arachnoid and dura mater coverings. An early idea concerning the creation of CSDH was that it was caused by a severe injury that caused the piercing of the bridge veins that connect the brain to the draining dural-venous sinuses.[
Each year, the incidence of CSDH ranges from 1 to 5.3 cases/100,000 people. The incidence of this condition is increasing due to an older population and medical conditions such as hemodialysis, anticoagulants, and/or antiplatelet medication.[
There are several routes by which blood or fluid might collect within the dural border cell layer. The most prevalent mechanism is head trauma, which produces bridging vein ripping and, as a result, acute subdural hematoma.[
Asymptomatic patients are often handled conservatively, with an observation period, medical care, intracranial pressure control, anticoagulant reversal, and serial examinations.[
MATERIALS AND METHODS
Protocol
We conducted a comprehensive meta-analysis and systematic review, which was previously registered on PROSPERO (CRD42023454162), to assess the efficacy of steroids in treating CSDH and its long-term outcomes. Our study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and the Cochrane Handbook of Systematic Review and Intervention.
Search strategy
We conducted a thorough literature search across electronic databases, including PubMed, Google Scholar, Medline, Embase, and Scopus. Our search included articles up until June 2023, using relevant keywords such as “steroids,” “dexamethasone (DXM),” “chronic subdural hematoma,” “CSDH,” and “chronic SDH.” The full mesh phrase has been separately provided in Supplementary File 1. Only the English language was used for the data search.
Eligibility criteria
We included studies that met specific criteria: Patients diagnosed with CSDH, patients receiving steroids orally or through other forms as monotherapy or adjuvant therapy, adults aged over 18, control group patients not using steroids, clearly defined primary and secondary outcomes, such as mortality, modified Rankin scale (mRS) 0–3, mRS 4–6, complications, and recurrence. Only randomized controlled trials (RCTs) or cohort studies were considered. We excluded studies that did not meet these criteria, such as those in languages other than English, studies involving non-human subjects, pediatric populations, studies lacking desired outcomes, and study types other than RCTs or cohorts (e.g.,case–control, case series, editorials, and single-arm studies).
Study screening and data extraction
Two reviewers independently screened articles. Articles meeting inclusion criteria were initially included, and subsequent exclusion was based on title, abstract, and full-text review. A third reviewer resolved any discrepancies or conflicts through mutual agreement. Authors’ names, publication year, and baseline characteristics, including demographic data (population age, CSDH follow-up, and sample size), intervention details (steroids duration and dosage), and outcome data (recurrence, mortality, and complication rate) were all extracted from relevant studies.
Outcomes
Primary outcomes assessed include mortality in patients treated with steroids only, steroids and surgery, and no steroids, as well as recurrence rate and mRS score at six months. Secondary outcomes encompass complications and hospital stays exceeding 30 days.
Data analysis and risk of bias
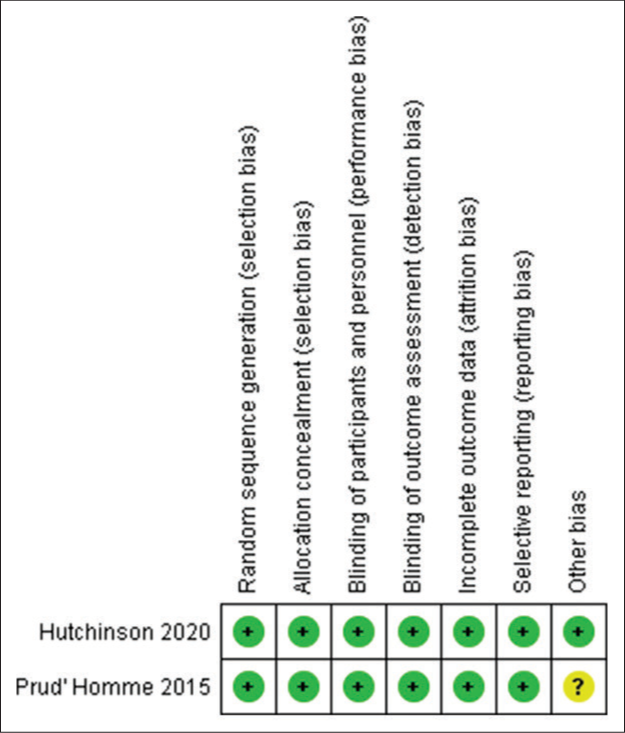
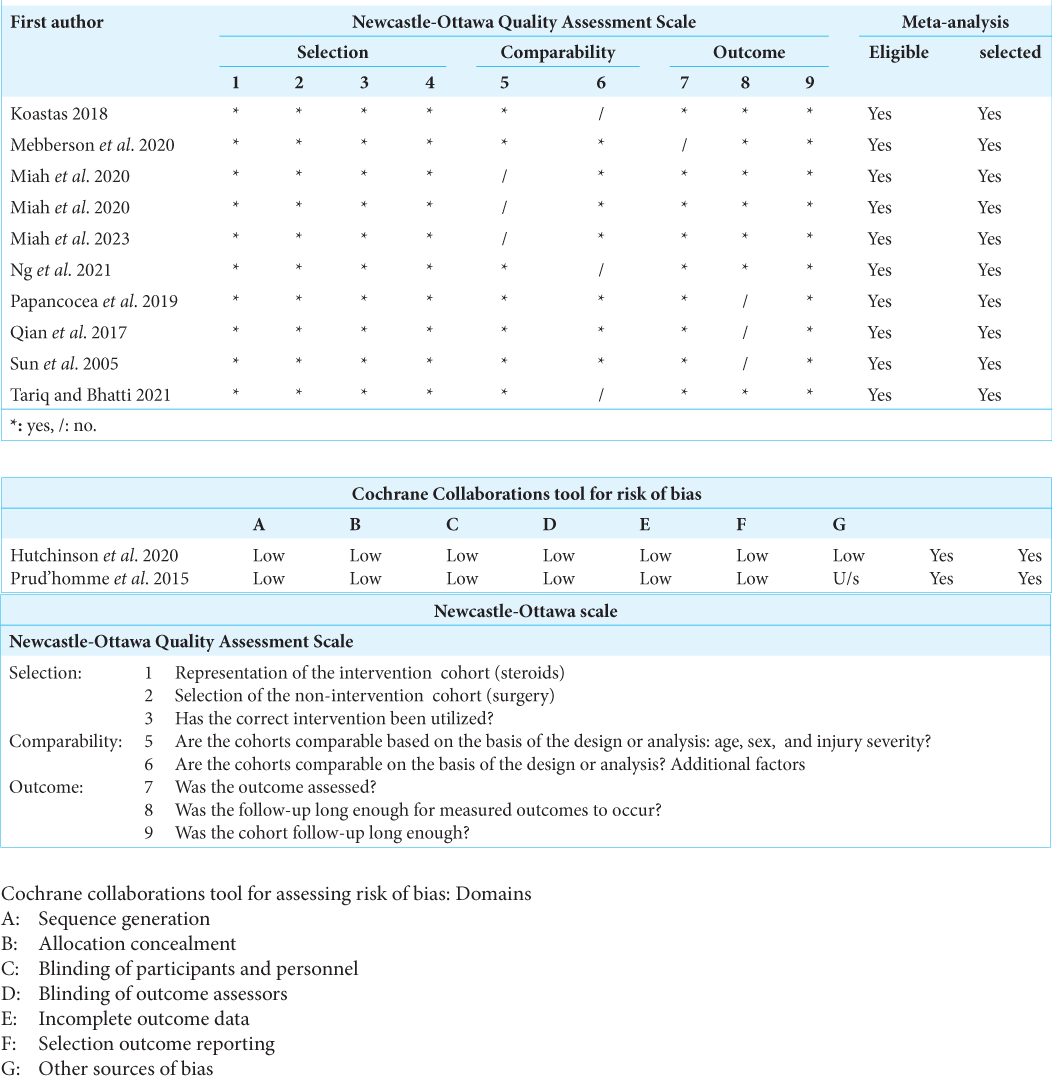
We analyzed the data using RevMan version 5.4 from the Cochrane Collaboration. Odds ratios (OR) were employed with a confidence interval of 95%, and a random or fixed effect model was selected and used. Funnel plots were created for primary and secondary outcomes using this model for mortality, mRS score, and complication rate. I2 statistic was used to assess heterogeneity among included studies. When I2 exceeded 50%, the heterogeneity was deemed significant, and sensitivity analysis was interpreted in light of the features of the study. Risk of Bias of included RCTs was assessed using the Cochrane tool for risk of bias. Studies were graded as low risk, high risk, and uncertain risk for selection bias, reporting bias, other bias, and performance bias. Observational studies and cohorts’ Risk of bias were interpreted using the Newcastle-Ottawa scale for selection, comparability, and outcome.
RESULTS
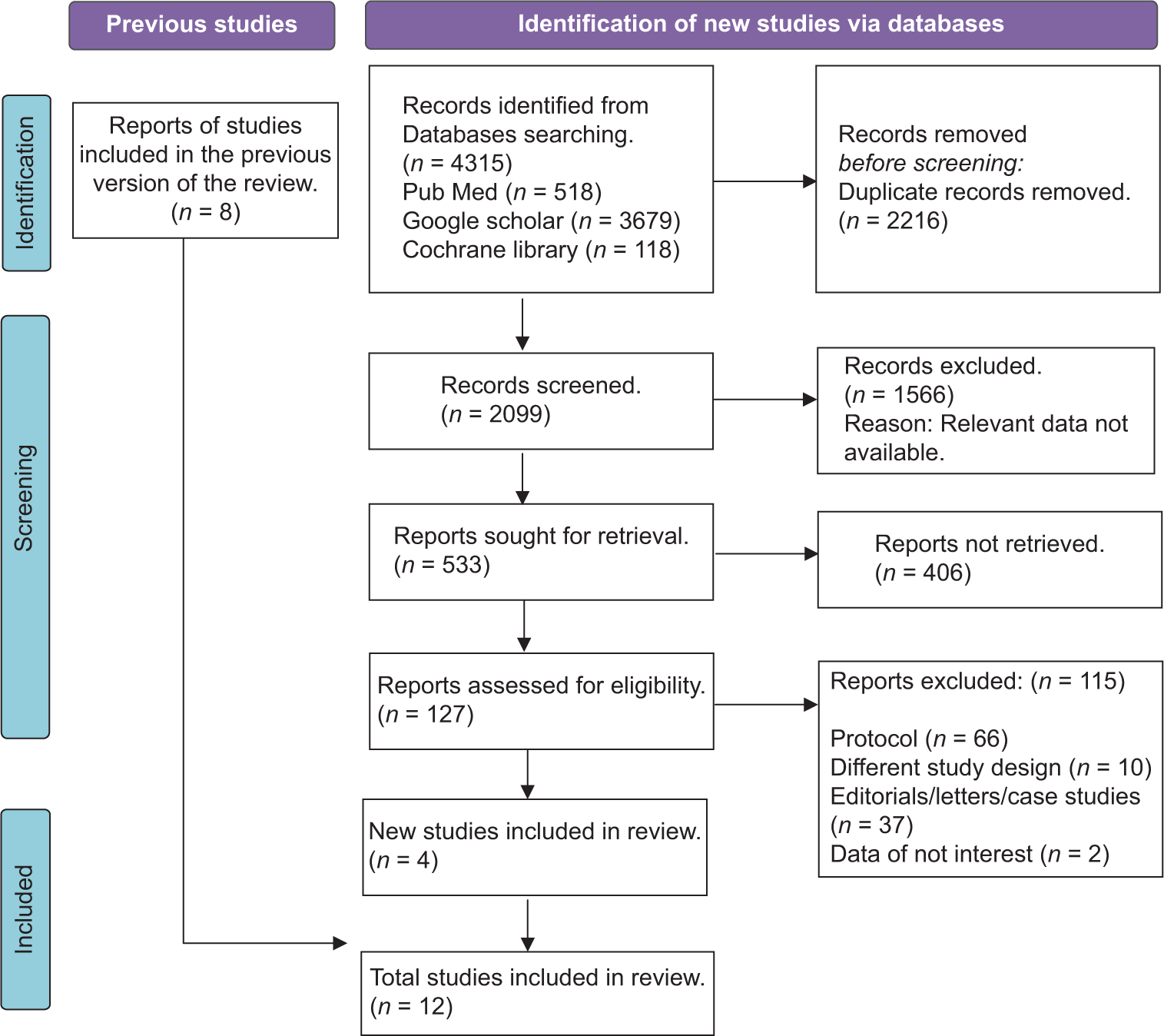
Literature search
The initial phase of the literature search yielded a pool of 4315 articles. After removing duplicates, 2099 unique records were left for the subsequent screening process. All 533 records underwent an initial assessment based on their titles and abstracts, leading to the identification of 127 articles for more in-depth evaluation. Following a rigorous examination, 115 studies were excluded due to a range of reasons, such as failure to meet inclusion criteria or the absence of relevant data. Ultimately, the meta-analysis focused on a total of 12 articles that met the predefined inclusion criteria [
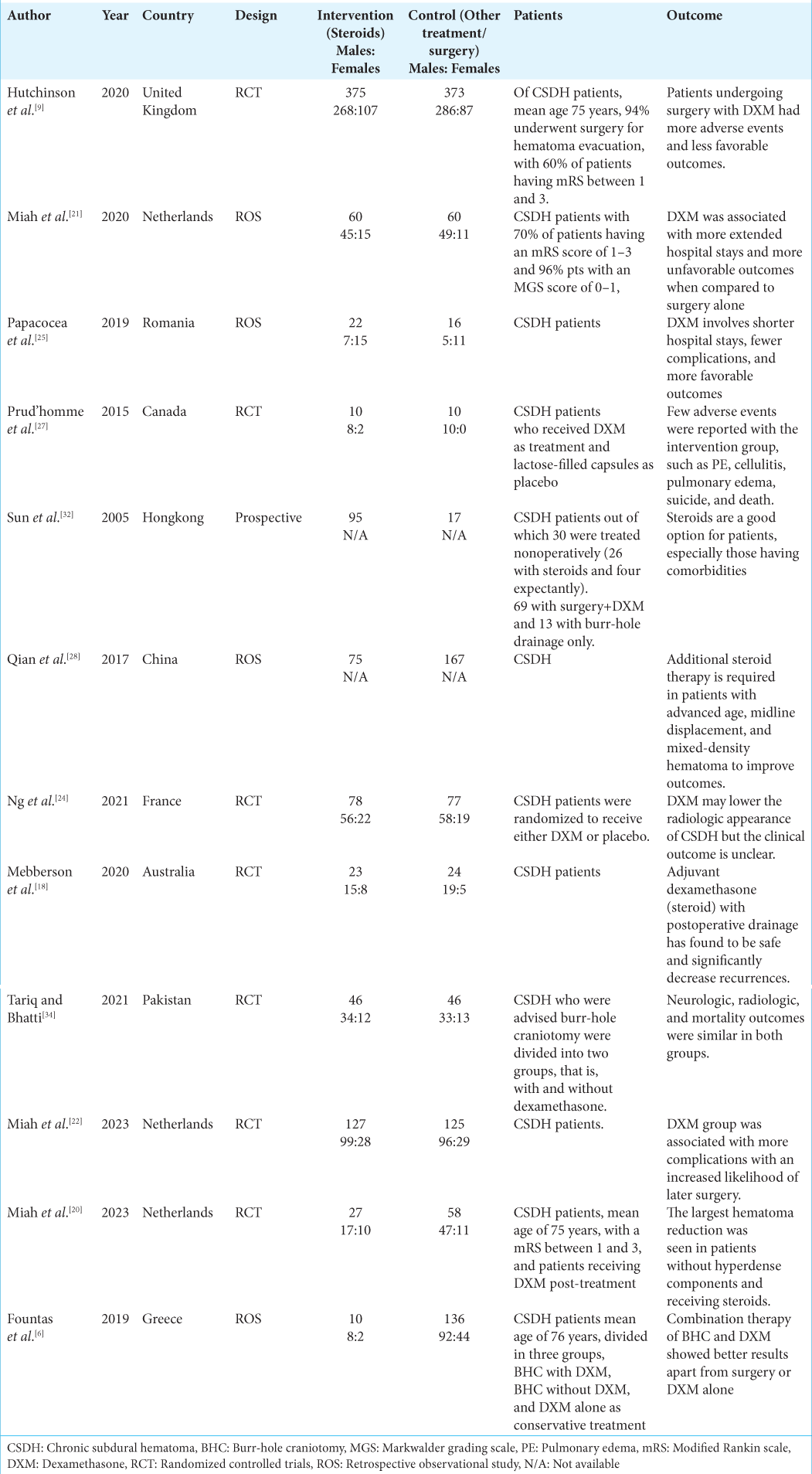
Study characteristics
All studies included focused on CSDH patients undergoing steroid intervention.[
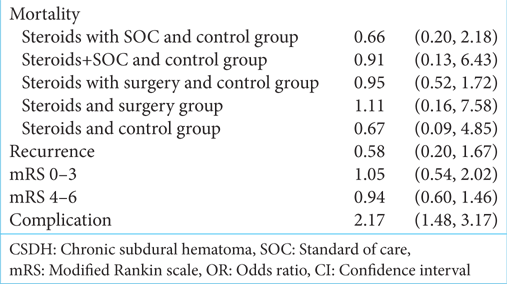
Mortality
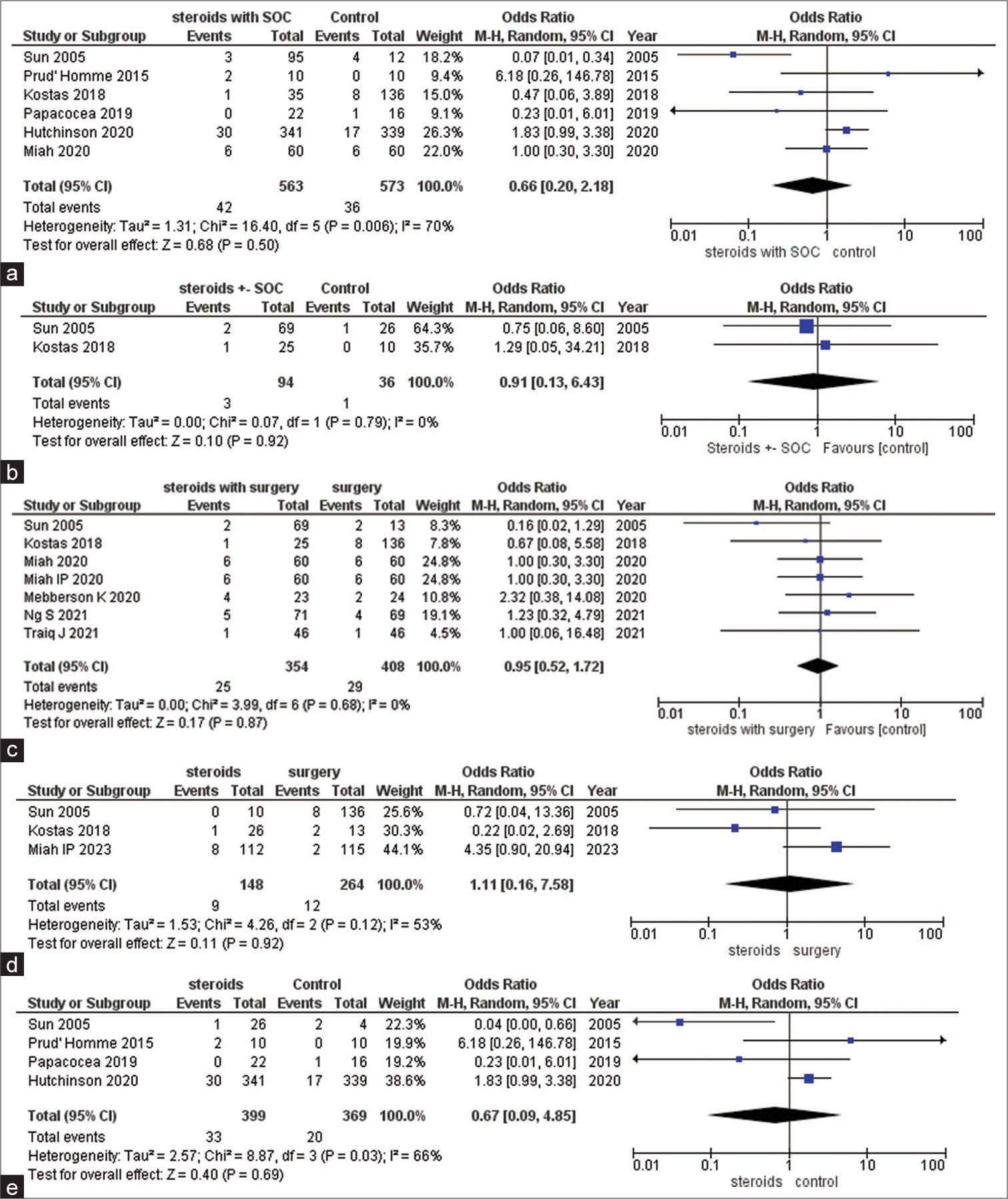
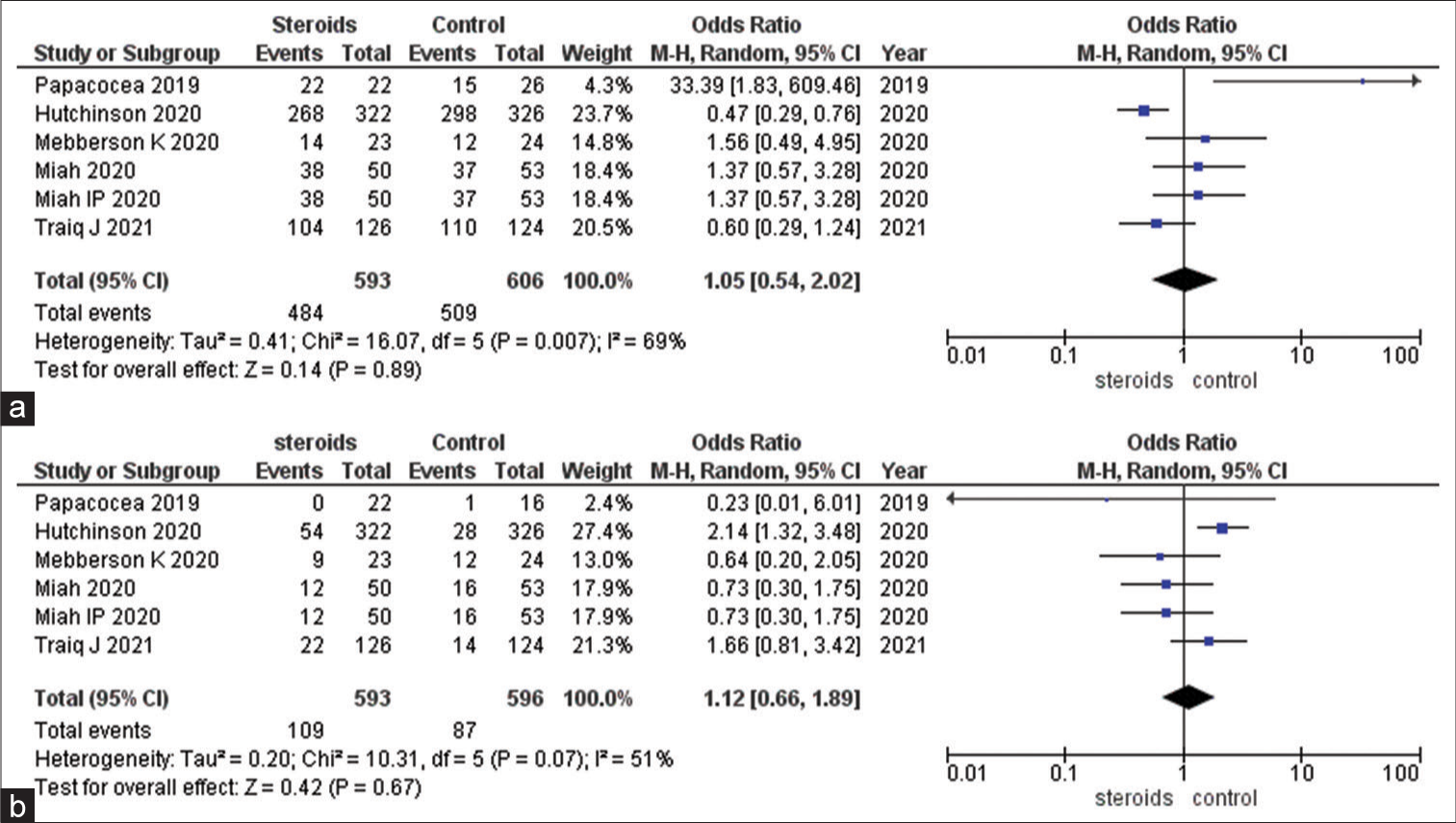
The comparison of steroids alongside standard of care (SOC) versus the control group yielded an OR of 0.66 (95% confidence interval [CI] 0.20, 2.18; I2 70%; P = 0.50), indicating a non-significant trend toward reduced mortality with steroids [
Figure 2:
(a) Forest plot demonstrating mortality outcome between steroids with standard of care (SOC) and control group. (b) Forest plot demonstrating mortality outcome between steroids ± SOC and control group. (c) Forest plot demonstrating mortality outcome between steroids with surgery and control. (d) Forest plot demonstrating mortality outcome between steroids and surgery group. (e) Forest plot demonstrating mortality outcome between steroids and control group. M-H: Mantel-Haenszel test, CI: Confidence interval.
Mortality outcome between different treatment regimes
In the exploration of various treatment strategies, the meta-analysis did not identify statistically significant differences in terms of mortality rates across different combinations of steroids, surgery, and standard care. The ORs, indicated by their respective confidence intervals, suggest a lack of definitive effect. The wide confidence intervals reflect the inherent uncertainty in these findings. While the analysis does not firmly establish distinct mortality effects associated with these diverse treatment regimens, it underscores the intricate and varied nature of treatment responses, as evidenced by the collected data. To provide a more comprehensive understanding, the odds ratios and their associated values for each comparison are as follows:
Steroids ± SOC versus control
The odds ratio is 0.91, indicating no significant difference (95% CI 0.13, 6.43; I2 0%; P = 0.92) [
Steroids with surgery versus surgery alone
The OR is 0.95, indicating no significant difference (95% CI 0.52, 1.72; I2 0%; P = 0.87) [
Steroids alone versus surgery alone
The odds ratio is 1.11, indicating no significant difference (95% CI 0.16, 7.58; I2 53%; P = 0.92) [
Steroids versus control
The OR is 0.67, indicating no significant difference (95% CI 0.09, 4.85; I2 66%; P = 0.69) [
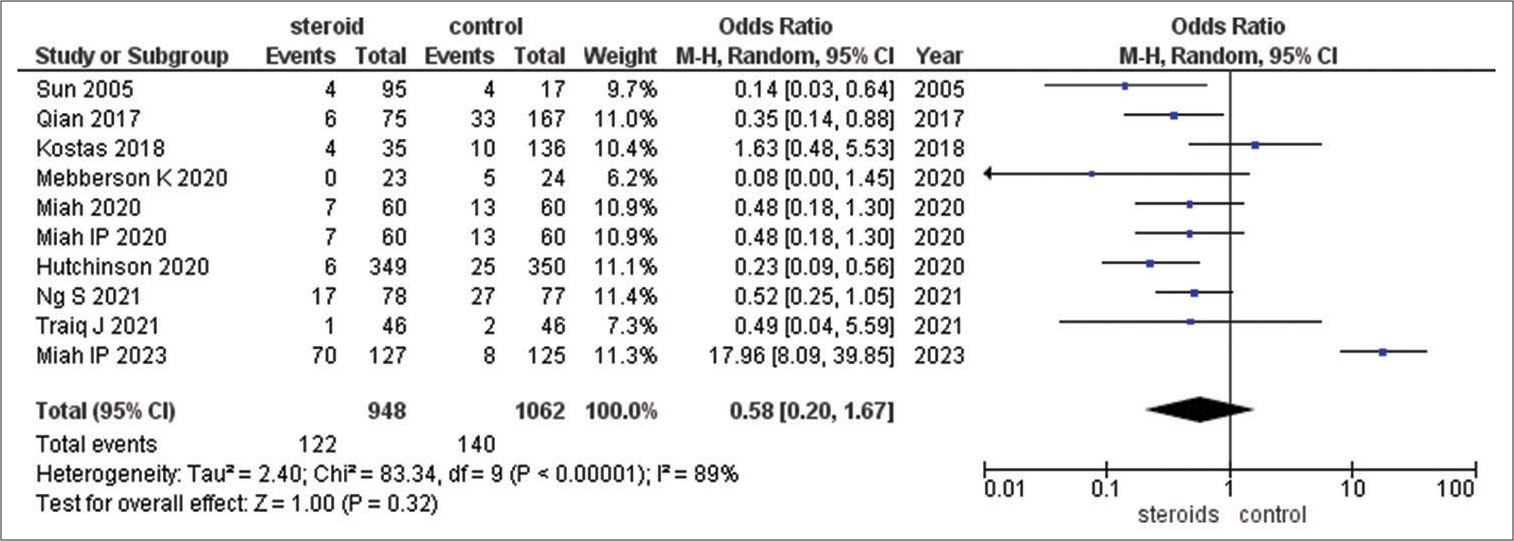
Recurrence of subdural hematoma
The use of steroids appeared to be associated with lower odds of recurrence of subdural hematoma compared to the control group, as suggested by the OR of 0.58. However, the wide CI (0.20–1.67) indicates uncertainty, and the high I2 value (89%) signifies substantial variability among the studies. Sensitivity analysis, which mitigates the impact of a specific study, yielded a more pronounced effect size (OR = 0.41), implying a potential beneficial effect of steroids in reducing recurrence risk [
mRS score at three months
Regarding functional outcomes at three months, as measured by mRS scores, the use of steroids did not exhibit a distinct advantage over the control group. The ORs for both mRS scores (0–3 and 4–6) hovered around 1, indicating no substantial difference. The wide CIs and moderate I2 values (69% and 51%) underscore variability and limited precision in these results. Sensitivity analysis produced minor adjustments to the ORs but did not reveal a strong, statistically significant effect [
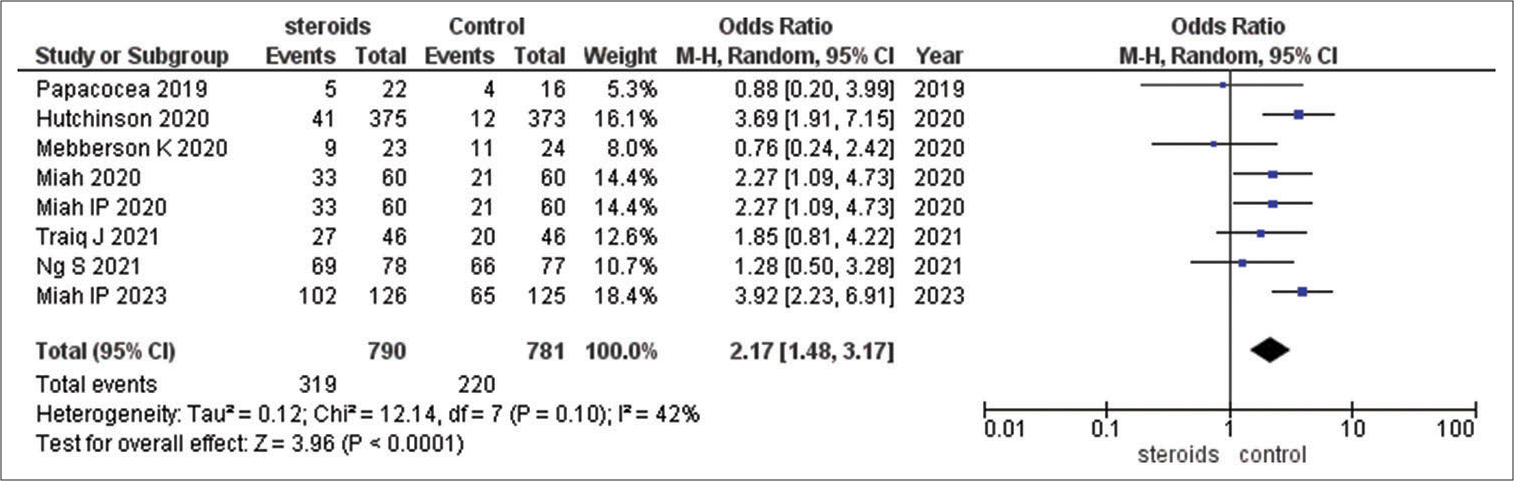
Occurrence of overall adverse effects/complications
Occurrence of overall adverse effects/complications between steroids versus control: OR = 2.17 (95% CI 1.48, 3.17; I2 42%; P < 0.0001). The analysis suggests a significant increase in adverse effects and complications with steroids [
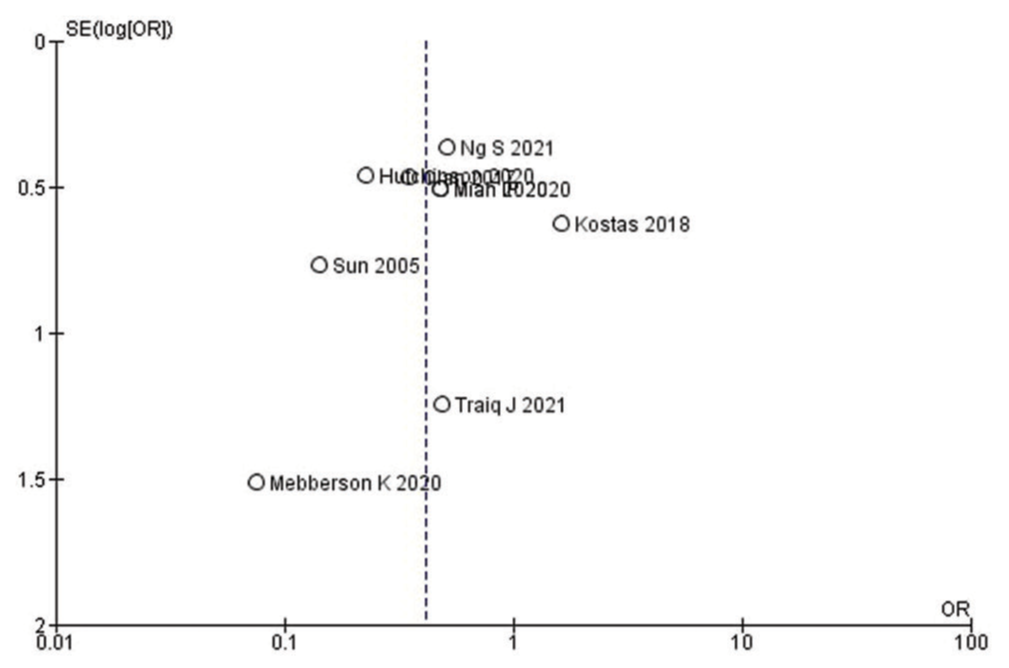
Risk of bias
Funnel plot was used for the assessment of publication bias for the recurrence outcome of the included studies [
DISCUSSION
Our study found significantly lower odds of recurrence of CSDH in the treatment group as compared to control group which was similar to Shrestha et al.,[
Our study suggests a significant increase in overall adverse effects and complications with steroids in treatment group as compared to control group. This finding is also evident in Kolias et al.[
There is no difference in the treatment success and neurologic outcome in the steroid group as compared to control group in our study. This is similar to Shrestha et al.[
The study Berghauser et al. demonstrated the therapeutic advantage of steroid therapy.[
Our Analysis findings point to a potential benefit of steroids in lowering the possibility of recurrence. According to the investigation, steroids have a markedly increased risk of side effects and problems. Because there is no advantage in terms of therapeutic success, mortality, or neurologic outcomes for individuals with subdural hematoma, the use of steroids is restricted to special cases.
The majority of the included studies were observational and nonrandomized, which increased the possibility of confounding and selection bias. In addition, there were heterogeneities in our study as a result of the population’s different baseline characteristics, different DXM treatment dosages in various trials, varying DXM treatment durations of 1–3 weeks in various studies, and various treatment modalities in control groups. Our meta-analysis has its limitations due to these heterogeneities. A subgroup analysis was carried out to address the variance in treatment modalities.
In a nutshell, the major findings of our study were decrease in the recurrence rate of CSDH in patients receiving DXM treatment as compared to control group and increase in the adverse effects or complications after DXM treatment. Due to significant heterogenicity among studies and various treatment regimes, we did not see any significant advantage in terms of mortality, treatment success, and mRS score at 3 months. These findings are somewhat similar to previous meta-analysis done by Shrestha et al.[
Limitations
After our thorough assessment, the shortcomings of the current corpus of research become evident. The methodology used in earlier research is one of the main obstacles. Our findings highlight the critical need for more meticulously designed cohort studies and RCTs. The validity and relevance of previous research are weakened by the dearth of well-designed studies. Moreover, we limited the scope of our literature review to adult populations with an age of 18 years or older. This criterion limited the generalizability of our findings, even if it was required for the particular scope of our review. Subgroups based on age, such teenagers and the elderly, are glaringly absent from the current body of research. Furthermore, the current research’s geographic reach is constrained. Since many research have only been carried out in certain areas or nations, extrapolating the results to a global setting can be difficult.
CONCLUSION
This meta-analysis delving into the application of steroids in CSDH sheds light on several pivotal clinical outcomes. While discernible trends suggestive of reduced mortality and recurrence rates were observed in conjunction with steroid use, these trends often fell short of statistical significance, primarily due to the extensive confidence intervals and significant study heterogeneity. Furthermore, the intricate interplay of distinct treatment protocols in influencing mortality outcomes was evident, as illustrated by the diverse ORs witnessed across various comparisons. The analysis also underscores the potential trade-off between favorable effects and an elevated likelihood of encountering adverse effects and complications linked to steroid administration. These outcomes underscore the necessity for continuous research to elucidate the precise impact of steroids within this context and underscore the significance of tailoring treatment decisions to encompass patient-specific attributes while taking into account the broader landscape of available evidence.
Authors’ contributions
AH did the conceptualization. MAS, SMSA, and MAR conducted the literature search and screening. Drafting of the manuscript and writing was done by ZUNM, AK, RN, and MSM. AH did the statistical analysis. TKFA performed the editing. All authors have read and agreed to the final version of the manuscript.
Ethics approval
Not required.
Availability of data and material
Not applicable.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY FILES
References
1. Ayub K, Yarnell O. Subdural haematoma after whiplash injury. Lancet. 1969. 294: 237-9
2. Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R. The role of corticosteroids in the management of chronic subdural hematoma: A systematic review. Eur J Neurol. 2012. 19: 1397-403
3. Coleman PL, Patel PD, Cwikel BJ, Rafferty UM, Sznycer-Laszuk R, Gelehrter TD. Characterization of the dexamethasone-induced inhibitor of plasminogen activator in HTC hepatoma cells. J Biol Chem. 1986. 261: 4352-7
4. Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Rodríguez-Salazar A, Galacho-Harriero AM, FernándezArconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur). 2009. 20: 346-59
5. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: Epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020. 141: 339-45
6. Fountas K, Kotlia P, Panagiotopoulos V, Fotakopoulos G. The outcome after surgical vs nonsurgical treatment of chronic subdural hematoma with dexamethasone. Interdiscip Neurosurg. 2019. 16: 70-4
7. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: Surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005. 107: 223-9
8. Glover D, Labadie EL. Physiopathogenesis of subdural hematomas. Part 2: Inhibition of growth of experimental hematomas with dexamethasone. J Neurosurg. 1976. 45: 393-7
9. Hutchinson PJ, Edlmann E, Bulters D, Zolnourian A, Holton P, Suttner N. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med. 2020. 383: 2616-27
10. Ivamoto HS, Lemos HP, Atallah AN. Surgical treatments for chronic subdural hematomas: A comprehensive systematic review. World Neurosurg. 2016. 86: 399-418
11. Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. Epidemiology of chronic subdural hematomas. No Shinkei Geka. 2011. 39: 1149-53
12. Kolias AG, Edlmann E, Thelin EP, Bulters D, Holton P, Suttner N. Dexamethasone for adult patients with a symptomatic chronic subdural haematoma (Dex-CSDH) trial: Study protocol for a randomised controlled trial. Trials. 2018. 19: 670
13. Kravtchouk AD, Likhterman LB, Potapov AA, El-Kadi H. Postoperative complications of chronic subdural hematomas: Prevention and treatment. Neurosurg Clin N Am. 2000. 11: 547-52
14. Krupa M. Chronic subdural hematoma: A review of the literature. Part 1. Ann Acad Med Stetin. 2009. 55: 47-52
15. Labadie EL, Glover D. Local alterations of hemostaticfibrinolytic mechanisms in reforming subdural hematomas. Neurology. 1975. 25: 669-75
16. Liu W, Bakker NA, Groen RJ. Chronic subdural hematoma: A systematic review and meta-analysis of surgical procedures. J Neurosurg. 2014. 121: 665-73
17. Markwalder TM. Chronic subdural hematomas: A review. J Neurosurg. 1981. 54: 637-45
18. Mebberson K, Colditz M, Marshman LA, Thomas PA, Mitchell PS, Robertson K. Prospective randomized placebo-controlled double-blind clinical study of adjuvant dexamethasone with surgery for chronic subdural haematoma with post-operative subdural drainage: Interim analysis. J Clin Neurosci. 2020. 71: 153-7
19. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J Clin Neurosci. 2018. 50: 7-15
20. Miah IP, Blanter A, Tank Y, Zwet EW, Rosendaal FR, Peul WC. Change in hematoma size after dexamethasone therapy in chronic subdural hematoma subtypes: A prospective study in symptomatic patients. J Neurotrauma. 2023. 40: 228-39
21. Miah IP, Herklots M, Roks G, Peul WC, Walchenbach R, Dammers R. Dexamethasone therapy in symptomatic chronic subdural hematoma (DECSA-R): A retrospective evaluation of initial corticosteroid therapy versus primary surgery. J Neurotrauma. 2020. 37: 366-72
22. Miah IP, Holl DC, Blaauw J, Lingsma HF, den Hertog HM, Jacobs B. Dexamethasone versus surgery for chronic subdural hematoma. N Engl J Med. 2023. 388: 2230-40
23. Missori P, Salvati M, Polli FM, Conserva V, Delfini R. Intraparenchymal haemorrhage after evacuation of chronic subdural haematoma. Report of three cases and review of the literature. Br J Neurosurg. 2002. 16: 63-6
24. Ng S, Boetto J, Huguet H, Roche PH, Fuentes S, Lonjon M. Corticosteroids as an adjuvant treatment to surgery in chronic subdural hematomas: A multi-center double-blind randomized placebo-controlled trial. J Neurotrauma. 2021. 38: 1484-94
25. Papacocea T, Popa E, Dana T, Papacocea R. The usefulness of dexamethasone in the treatment of chronic subdural hematomas. Farmacia. 2019. 67: 140-5
26. Petralia CC, Manivannan S, Shastin D, Sharouf F, Elalfy O, Zaben M. Effect of steroid therapy on risk of subsequent surgery for neurologically stable chronic subdural hemorrhage-retrospective cohort study and literature review. World Neurosurg. 2020. 138: e35-41
27. Prud’homme M, Mathieu F, Marcotte N, Cottin S. A pilot placebo controlled randomized trial of dexamethasone for chronic subdural hematoma. Can J Neurol Sci. 2016. 43: 284-90
28. Qian Z, Yang D, Sun F, Sun Z. Risk factors for recurrence of chronic subdural hematoma after burr hole surgery: Potential protective role of dexamethasone. Br J Neurosurg. 2017. 31: 84-8
29. Shi M, Xiao LF, Zhang TB, Tang QW, Zhao WY. Adjuvant corticosteroids with surgery for chronic subdural hematoma: A systematic review and meta-analysis. Front Neurosci. 2021. 15: 786513
30. Shrestha DB, Budhathoki P, Sedhai YR, Jain S, Karki P, Jha P. Steroid in chronic subdural hematoma: An updated systematic review and meta-analysis post DEX-CSDH trial. World Neurosurg. 2022. 158: 84-99
31. Stroobandt G, Fransen P, Thauvoy C, Menard E. Pathogenetic factors in chronic subdural haematoma and causes of recurrence after drainage. Acta Neurochir (Wien). 1995. 137: 6-14
32. Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: Preliminary results of using dexamethasone. Br J Neurosurg. 2005. 19: 327-33
33. Tang G, Chen J, Li B, Fang S. The efficacy of adjuvant corticosteroids in surgical management of chronic subdural hematoma: A systematic review and meta-analysis. Front Neurol. 2021. 12: 744266
34. Tariq J, Bhatti SN. Adjunctive postoperative course of dexamethasone in chronic subdural hematoma: Effect on surgical outcome. Pak J Med Sci. 2021. 37: 1877-82
35. Wang D, Gao C, Xu X, Chen T, Tian Y, Wei H. Treatment of chronic subdural hematoma with atorvastatin combined with low-dose dexamethasone: Phase II randomized proof-of-concept clinical trial. J Neurosurg. 2020. 134: 235-43
36. Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg. 2016. 11: 330-42
37. Yamashima T, Friede R. Why do bridging veins rupture into the virtual subdural space?. J Neurol Neurosurg Psychiatry. 1984. 47: 121-7
38. Yao Z, Hu X, Ma L, You C. Dexamethasone for chronic subdural haematoma: A systematic review and meta-analysis. Acta Neurochir (Wien). 2017. 159: 2037-44
39. Zhao Y, Xiao Q, Tang W, Wang R, Luo M. Efficacy and safety of glucocorticoids versus placebo as an adjuvant treatment to surgery in chronic subdural hematoma: A systematic review and meta-analysis of randomized controlled clinical trials. World Neurosurg. 2022. 159: 198-206.e4