- Department of Neurosurgery, Donald and Barbara Zucker School of Medicine, Hempstead, United States
- Department of Neurosurgery, North Shore University Hospital, Manhasset, United States.
Correspondence Address:
Michael Ohene-Adjei, Fourth Year Medical Student, Department of Neurosurgery, Donald and Barbara Zucker School of Medicine, 500 Hofstra Blvd, Hempstead, NY 11549, United States.
DOI:10.25259/SNI_409_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael Ohene-Adjei1,2, Sabrina Leone Begley1,2, Richard Temes2, Michael Schulder2. Efficacy of continuous electroencephalogram for the management of altered mental status in the neurosurgical intensive care unit. 07-Jul-2023;14:235
How to cite this URL: Michael Ohene-Adjei1,2, Sabrina Leone Begley1,2, Richard Temes2, Michael Schulder2. Efficacy of continuous electroencephalogram for the management of altered mental status in the neurosurgical intensive care unit. 07-Jul-2023;14:235. Available from: https://surgicalneurologyint.com/surgicalint-articles/12395/
Abstract
Background: Continuous electroencephalograms (cEEGs) are often used in the neurosurgical intensive care unit (NSICU) to detect subclinical seizures (SCSs) in patients with altered mental status (AMS). This retrospective study evaluated the efficacy of this approach for improving patient outcomes.
Methods: We reviewed the records of 100 patients admitted to the NSICU between 2015 and 2020 who underwent continous electroencephalograms (cEEG) during workup of unexplained AMS. Patient outcomes were classified as positive (discharged), neutral (transfer of care), or negative (dead). Incidence of SCSs on cEEG and association with patient outcomes was analyzed with Chi-square analysis and relative risk (RR).
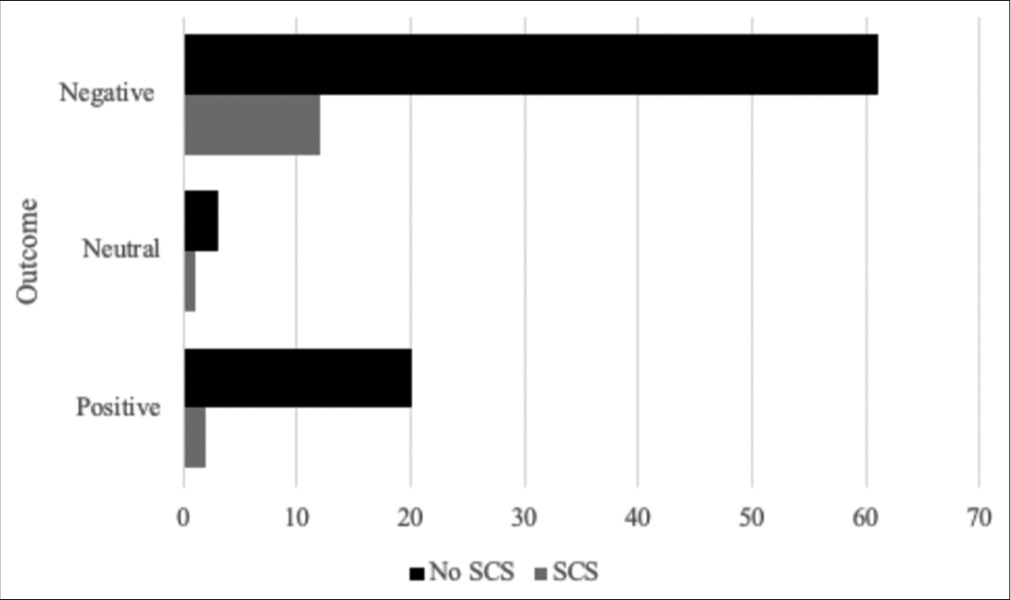
Results: For the 99 included patients, median age was 62 years and 43% were female. About 15.2% had a known or newly diagnosed brain tumor. Outcomes were positive in 22 patients, neutral in four, and negative in 73. SCSs were detected in 15 patients, of whom 12 died, two were discharged, and one whose care was transferred. Chi-square association between SCS and outcome (P = 0.59) and RR of death associated with SCS diagnosis (1.1) was not significant.
Conclusion: We found a lower incidence of SCSs (15.2%) than reported in the literature. In the absence of clinically evident seizures, continous cEEGs performed in the NSICU to determine the etiology of AMS did not yield an improvement in patient outcomes, and patients diagnosed and treated for SCS did not have statistically decreased risk of death. In summary, electroencephalogram monitoring for SCS is important but should not delay diagnosis and treatment of other, potentially life-threating etiologies of AMS.
Keywords: Altered mental status, Electroencephalography, Outcome, Subclinical seizures, Treatment
INTRODUCTION
Altered mental status (AMS) is common in patients presenting to the emergency department, admitted to the hospital,[
When these standard evaluations have been exhausted yet AMS persists, continuous electroencephalogram (cEEG) can be used to rule out nonconvulsive seizures, otherwise known as subclinical seizures (SCSs).[
While previous studies have looked at the general use of cEEG for NSICU patients and detecting SCSs, none have questioned how either the administration or results of cEEGs in these patients have affected patient outcomes. We hypothesize that an over-reliance on and prioritization of cEEG results may lead to other causes of AMS being missed or necessary treatment being delayed with no actual improvement in patient outcomes.
MATERIALS AND METHODS
Patient selection
This is a retrospective chart review that was approved by the Northwell Health Institutional Review Board after expedited review (IRB 20-0546) and informed consent was waived. Patients who were admitted to the North Shore University Hospital NSICU between 2015 and 2020 were identified using the Sunrise electronic health record and screened for eligibility. We included adult patients with AMS of no apparent cause documented on their medical record, and who underwent cEEG in their workup. Patients were excluded if they did not receive cEEG monitoring, had clinical seizure activity, or lacked an official EEG report or documented results. The hospital course of these patients was then tracked for any changes in treatment and their ultimate outcome.
Statistical analysis
Patient outcomes were stratified into three categories: positive, neutral, and negative. Positive outcomes were discharges to home or to a lower acuity rehabilitation center; neutral outcomes involved the transfer of care to another hospital; and negative outcomes included death or decision for palliative care during hospitalization. A Chi-square test was performed to determine any association between the presence of the SCSs and treatment outcomes with a statistical level of P = 0.05. Positive and neutral outcomes were combined to formulate a 2 × 2 table to calculate the relative risk (RR) of a negative outcome in patients with and without SCSs.
RESULTS
Our initial search resulted in 100 eligible patients. One patient was excluded for a diagnosis of generalized tonic clonic seizures without SCSs. The final cohort of 99 patients had a median age of 64 ± 16. About 43.4% of patients were female and 56.6% were male [
The association between the presence of a SCS and treatment outcome was not significant (χ2 [
Case illustrations
Delay in care due to cEEG
A 48-year-old woman with metastatic ovarian carcinoma was admitted to the NSICU after a left medial frontal metastasis was resected through a transfalcine approach. She remained neurologically intact postoperatively and for the next 24 h. She then developed progressively decreasing level of consciousness necessitating intubation for airway protection. Her EEG, although appropriately ordered by the neurointensive care team, was prematurely read as showing NCSE. Subsequently her AED dosages were increased and she remained on cEEG. Despite this change in management, there was no improvement in her mental status. After discontinuation of the cEEG, subsequent imaging was acquired and showed significant bifrontal swelling. She underwent emergency bifrontal craniectomy and experienced complete neurological recovery, with cranioplasty 2 weeks later. Official interpretation of the cEEG by the attending neurologist, read 1 day after the craniectomy, described no seizure activity. After discharge, this patient lived 3 more years before dying of progressive ovarian cancer.
Positive SCS and discharged patient
A 78-year-old man 3-week post burr hole evacuation of a left frontal subdural hemorrhage was found to have stable reaccumulation of his subdural hematoma (SDH) without symptoms. Two days later, he presented with new-onset dysarthria and global aphasia. There had been no head trauma, falls, headaches, vomiting, vision problems, or seizures since his surgery. Noncontrast computed tomography (CT) demonstrated a stable SDH unchanged from his most recent CT 1 day prior. The next day, he received a cEEG showing a SCS and was started on lacosamide without significant change in mental status. The following day, repeat noncontrast CT of the head demonstrated an increased left subacute SDH now with a 6 mm midline shift and mass effect on lateral ventricles. He subsequently underwent a craniectomy followed by interval cranioplasty several days later. His mental status improved, he was deemed clinically stable and was discharged to an acute rehab facility.
DISCUSSION
The two aims of this study were (1) to determine the actual incidence of SCSs in our institution’s NSICU and (2) to evaluate whether the detection of an SCS with a cEEG played any significant role in the treatment and outcomes of those patients. The 15.2% incidence of SCSs in our sample was lower than previously reported in the literature.[
Even with a lower incidence of SCSs than expected, we found no significant association between the presence of SCSs and patient outcome. This suggests that in a patient with unexplained AMS, diagnosis and treatment of SCS did not correspond with patient outcome, whether it was improvement or death. Fourteen of the 15 patients with SCSs had either a tumor, an intracranial hemorrhage, or a history of epileptic seizures. It is worth noting that AED use is routine for 1 week after craniotomy for any reason, for patients with SAH, and patients who have sustained a head injury. The one patient with a stroke, AMS, and SCS died during their hospitalization. In addition, one of the hemorrhage patients, despite being treated appropriately for SCS, did not improve until after neurosurgical intervention for worsening SDH.
Another consideration that accompanies the diagnosis of a SCS is subsequent pharmacological management. Using intravenous anticonvulsants to treat SCSs and NSCE in patients with AMS is currently controversial due to the adverse effects of these drugs.[
Furthermore, there are a multitude of other causes of AMS in patients in the NSICU. These can be both life-threatening and acute in onset. Persistent AMS may not always be attributed to one, continuous cause. For example, a patient that presents with AMS and is diagnosed with a SCS (Illustrative case 2) is not precluded from experiencing other new or concurrent etiologies of AMS. Patients in the NSCU, as the name indicates, are in a critical and constantly changing condition. While cEEG is vital to diagnosing SCS, this should not occur at the expense of ceasing investigation into other causes and potentially delaying the diagnosis of a life-threating condition due to an overly high reliance on cEEG monitoring.
Limitations
Limitations of this study include it being a retrospective review that may have missed other patients who during this time period had cEEG or excluded patients who did not have complete EEG reports. Our ability to confirm the true incidence was limited by the number of patients with available and complete cEEG monitoring data. Due to the varying sample sizes and heterogeneous populations from the studies reported in the literature, a more conclusive large-scale study may be necessary to determine the true incidence of SCSs in NSICUs. Furthermore, it should be noted that a cEEG is a noninvasive test and the direct downsides of doing this test are primarily added time and cost, with generally lower risk then surgical or endovascular interventions.
CONCLUSION
Overall, our findings in this study challenge priorities in the standard workup of AMS in the NSICU. At present, cEEGs in the NSICU are commonly used to rule out SCSs in patients with AMS. Our results show that patient outcome was not affected by detection of a seizure and in some cases definitive treatment was actually delayed due to the prioritization of completing and reading EEG studies. We emphasize that focusing on cEEG as both a “knee-jerk” reaction and “end-all.”
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adam VN. Altered mental status in intensive care unit patients. Acta Med Croatica. 2012. 66: 3-6
2. American College of Emergency Physicia. Clinical policy for the initial approach to patients presenting with altered mental status. Ann Emerg Med. 1999. 33: 251-81
3. Behrouz R, Godoy DA, Azarpazhooh MR, Di Napoli M. Altered mental status in the neurocritical care unit. J Crit Care. 2015. 30: 1272-7
4. Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004. 62: 1743-8
5. Guibert TE, Bengoa NG, Sanchez CS, Navarro SJ, Ruiz IG, Sarasola MA. S22. Value of emergent EEG in the diagnosis and management of patients with acute altered mental status. Clin Neurophysiol. 2018. 129: e150
6. Henry TR, Ezzeddine MA. Approach to the patient with transient alteration of consciousness. Neurol Clin Pract. 2012. 2: 179-86
7. Jordan KG. Continuous EEG and evoked potential monitoring in the neuroscience intensive care unit. J Clin Neurophysiol. 1993. 10: 445-75
8. Kaplan PW. Prognosis in nonconvulsive status epilepticus. Epileptic Disord. 2000. 2: 185-93
9. Kubota Y, Nakamoto H, Egawa S, Kawamata T. Continuous EEG monitoring in ICU. J Intensive Care. 2018. 6: 39
10. Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: Clinical features and outcome. Arch Neurol. 2004. 61: 1090-4
11. Patti L, Gupta M, editors. Change in mental status. StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441973 [Last accessed on 2022 July 09]
12. Veauthier B, Hornecker JR, Thrasher T. Recent-onset altered mental status: Evaluation and management. Am Fam Physician. 2021. 104: 461-70
13. Walker MC. Treatment of nonconvulsive status epilepticus. Int Rev Neurobiol. 2007. 81: 287-97
14. Ziai WC, Schlattman D, Llinas R, Venkatesha S, Truesdale M, Schevchenko A. Emergent EEG in the emergency department in patients with altered mental states. Clin Neurophysiol. 2012. 123: 910-7