- Department of Surgery, College of Medicine, King Faisal University, AlAhsa, Saudi Arabia
- Department of Surgery, College of Medicine, Majmaah University, Majmaah, Saudi Arabia
- Department of Surgery, College of Medicine, Taibah University, Madinah, Saudi Arabia
- Department of Surgery, College of Medicine, Prince Sattam bin Abdulaziz University, Riyadh, Saudi Arabia
- Department of Surgery, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- Department of Surgery, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- Department of Surgery, College of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- Department of Surgery, Royal College of Surgeons in Ireland, Dublin, Ireland
- Department of Surgery, College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 0Department of Surgery, College of Medicine and Medical Science, Arabian Gulf University, Manama, Bahrain
- 1Department of Neurosurgery, King Fahad Hospital, AlAhsa, Saudi Arabia
Correspondence Address:
Saud Nayef Aldanyowi, Department of Surgery, College of Medicine, King Faisal University, AlAhsa, Saudi Arabia.
DOI:10.25259/SNI_502_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdulrahim Saleh Alrasheed1, Mohammed Abdullah Alqadhibi2, Rammaz Hussam Khoja3, Abdulaziz Saad Alayyaf4, Duaa Saleh Alhumoudi5, Mubarak Ibrahim Aldawlan6, Bedoor Obidallah Alghanmi7, Fahad Salman Almutairi8, Mohammed Ali Bin-Mahfooz9, Lina Abdulrahim Altalhi10, Saud Nayef Aldanyowi1, Abdulsalam Mohammed Aleid1, Awn Abdulmohsen Alessa11. Emerging therapies for immunomodulation in traumatic brain injury: A systematic review and meta-analysis. 13-Sep-2024;15:327
How to cite this URL: Abdulrahim Saleh Alrasheed1, Mohammed Abdullah Alqadhibi2, Rammaz Hussam Khoja3, Abdulaziz Saad Alayyaf4, Duaa Saleh Alhumoudi5, Mubarak Ibrahim Aldawlan6, Bedoor Obidallah Alghanmi7, Fahad Salman Almutairi8, Mohammed Ali Bin-Mahfooz9, Lina Abdulrahim Altalhi10, Saud Nayef Aldanyowi1, Abdulsalam Mohammed Aleid1, Awn Abdulmohsen Alessa11. Emerging therapies for immunomodulation in traumatic brain injury: A systematic review and meta-analysis. 13-Sep-2024;15:327. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13097
Abstract
Background: Traumatic brain injury (TBI) represents a significant global health burden, often leading to significant morbidity and mortality. Mounting evidence underscores the intricate involvement of dysregulated immune responses in TBI pathophysiology, highlighting the potential for immunomodulatory interventions to mitigate secondary injury cascades and enhance patient outcomes. Despite advancements in treatment modalities, optimizing therapeutic strategies remains a critical challenge in TBI management. To address this gap, this systematic review and meta-analysis aimed to rigorously evaluate the efficacy and safety of emerging immunomodulatory therapies in the context of TBI.
Methods: We searched electronic databases such as PubMed, Scopus, Web of Science and CENTRAL for relevant studies investigating the efficacy of immunomodulatory therapies in TBI that were meticulously selected for inclusion. Two independent reviewers meticulously performed data extraction and quality assessment, adhering to predefined criteria. Both randomized controlled trials (RCTs) and observational studies reporting clinically relevant outcomes, such as mortality rates, the Glasgow coma scale, and adverse events, were meticulously scrutinized. Meta-analysis techniques were employed to assess treatment effects across studies quantitatively and analyzed using the Review Manager software (version 5.2).
Results: Fourteen studies (n = 1 observational and n = 13 RCTs) were included in our study. Meta-analysis showed no significant overall mortality difference, but erythropoietin (EPO) significantly reduced mortality (odds ratio = 0.49; 95% confidence interval: 0.31–0.78, P = 0.002). The adverse event meta-analysis revealed no significant differences.
Conclusion: Immunomodulatory therapies did not significantly affect overall mortality, but EPO demonstrated promising results. Adverse events did not significantly differ from controls. Further research is warranted to refine TBI treatment protocols.
Keywords: Erythropoietin, Immunomodulatory therapies, Traumatic brain injury, Treatment outcomes
INTRODUCTION
Traumatic brain injury (TBI) can be defined as the disruption in brain function or other evidence of brain pathology caused by an external physical force. The yearly incidence of TBI is estimated at 50 million cases worldwide; thus, approximately half of the global population will have an episode of TBI in their life.[
A large number of TBI patients are living with lifelong motor, cognitive, and other disabilities. In addition, TBI increases the incidence of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease, among survivors.[
The severity of a TBI does not depend solely on the direct initial injury. The acute primary insult to the brain elicits a cascade of immune responses in the lesioned brain, which may induce secondary injuries and expand brain lesion size at the subacute stage of TBI. Indeed, prolonged immune responses could be observed in human and mouse brains years after TBI.[
Immunomodulation is the process of modifying the immune response to achieve a therapeutic effect. In the context of TBI, immunomodulation is used to reduce the damaging inflammatory response that occurs after the initial injury. One promising approach to achieve immunomodulation in TBI is through the use of mesenchymal stem cells (MSCs). MSCs have been shown to reduce secondary neurodegeneration and neuroinflammation, promote neurogenesis and angiogenesis, and improve functional outcomes in animal models of TBI.[
Researchers have shown substantial interest in understanding the use of anti-inflammatory drugs to address the robust inflammatory response observed during the acute phase of TBI. This inflammatory response has been shown to exacerbate secondary injury progression. Various categories of anti-inflammatory medications, such as non-steroidal anti-inflammatory drugs, corticosteroids, and minocycline, have been subject to scrutiny for their potential efficacy in managing TBI.[
Erythropoietin (EPO) was investigated as a potential treatment modality for TBI due to its neuroprotective properties. EPO is a glycoprotein hormone that regulates red blood cell production and has been shown to protect tissues in addition to its hematopoietic function. Research suggests that EPO may exert neuroprotective effects by reducing inflammation, preventing neuronal damage, promoting neurogenesis, and enhancing angiogenesis. These mechanisms could potentially help mitigate the secondary injury cascade that occurs following TBI, thereby improving outcomes for patients.[
The immunomodulation target therapies are showing great potential in managing TBI; as a result, we present a comprehensive systematic review and meta-analysis aimed at determining the efficacy and safety of emerging immunomodulation therapies in TBI. We aimed to better understand the impact of immunomodulatory interventions on key outcomes such as neurological function, mortality rates, and adverse events by systematically synthesizing data from relevant clinical studies.
MATERIALS AND METHODS
This study was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement. The current study was prospectively registered into the International Prospective Register of Systematic Reviews (PROSPERO) under the following protocol number: [CRD42024523787].
Study selection
Literature search strategy
We searched electronic databases such as PubMed, CENTRAL, Scopus, and Web of Science for relevant studies. We used the following search strategy: (Traumatic Brain Injury OR TBI) AND (Stem Cell OR Cyclosporin OR Erythropoietin OR Progesterone OR Immunomodulation OR Immunomodulatory Therapy).
Eligibility criteria
Studies were eligible for inclusion if they met the following criteria:
Studies published in English Population: Adult patients (≥18 years) were diagnosed with TBI Intervention: Immunomodulation therapies Comparator: Placebo Outcome: Studies reporting at least the mortality rate Study design: All randomized controlled trials (RCTs) and observational studies, either case–control or cohort studies.
Reviews, case reports, editorial letters, conference abstracts and study protocols, animal and phantom studies were excluded from the study.
Study selection process
Two independent reviewers screened the titles and abstracts of the retrieved articles to identify potentially relevant studies. Full-text articles were then reviewed for eligibility based on the inclusion and exclusion criteria. Discrepancies between reviewers were resolved through consensus or consultation with a third reviewer.
Data extraction
A standardized data extraction sheet was developed to capture relevant information from the included studies using Microsoft Excel. Each author extracted the following data from included studies: name of the first author, publication year, type of study, number of enrolled patients, sex ratio, period of the study, inclusion criteria, average ages, intervention details, adverse events, and outcomes. Two senior authors reviewed the extracted data. Any disagreements were resolved by group discussion.
Quality assessment
The methodological quality and risk of bias (ROB) of the included studies were assessed using appropriate tools based on the study design. For RCTs, the Cochrane ROB Tool was used to evaluate random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other sources of bias. Observational studies were assessed using the Newcastle-Ottawa Scale (NOS) for cohort and case–control studies.[
Data synthesis and analysis
Meta-analysis was conducted to pool data from included studies using appropriate statistical methods using the Review Manager software 5.1.4, provided by Cochrane Collaboration. Effect sizes were calculated for relevant outcomes, such as mortality rates, Glasgow coma scale (GCS), and adverse events. Random-effect models were used to account for heterogeneity between studies. Subgroup analyses were conducted to investigate potential sources of variability in the study outcomes, with a focus on the different types of immunomodulatory therapies utilized and the GCS scores as indicators of injury severity. The funnel plot and Egger regression test were used to assess potential publication bias [
RESULTS
Literature search
The initial database search yielded a total of 12561 articles. 4816 records were screened by titles and abstracts, and 150 articles were identified as potentially relevant for full-text review. Following a thorough assessment of eligibility criteria, 13 studies were included in the final analysis. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart, shown in
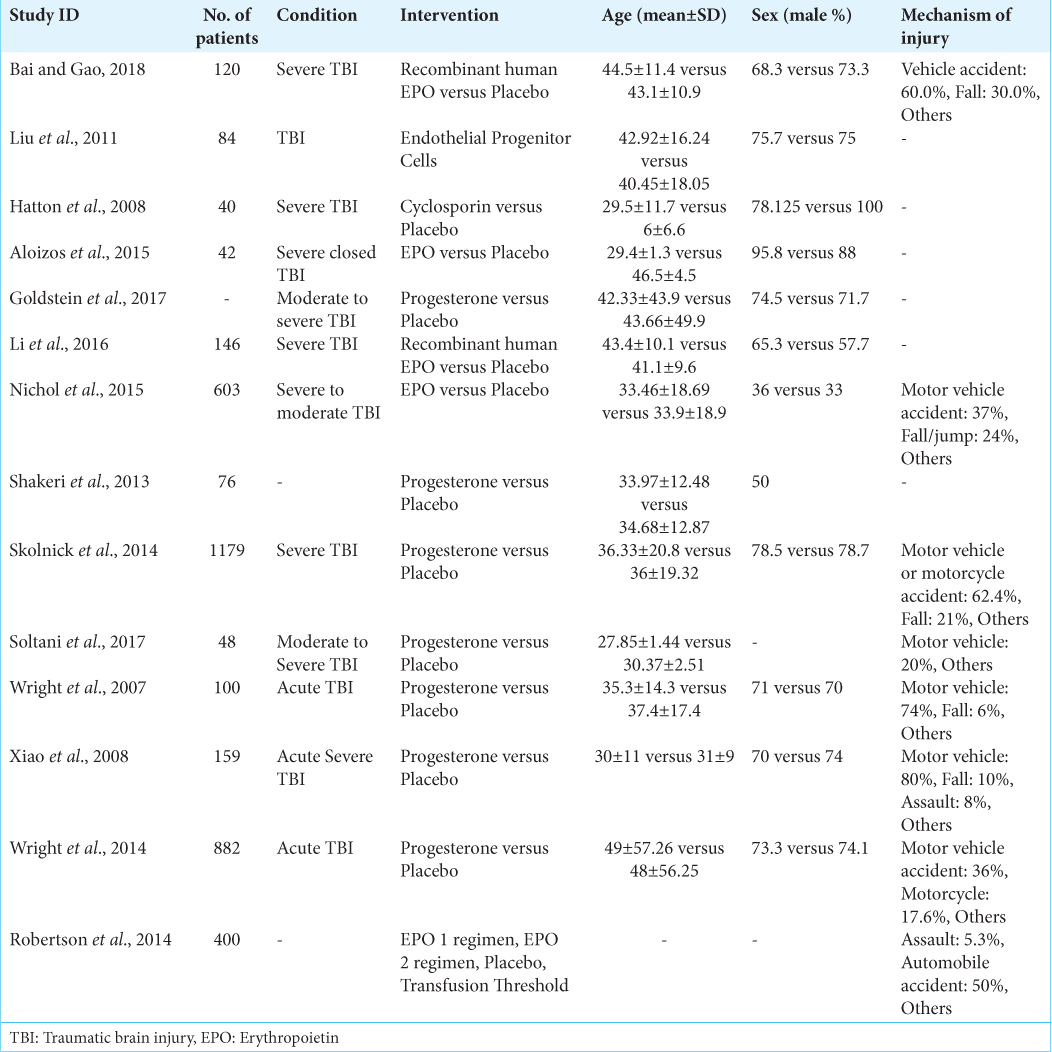
Characteristics of the included studies
There were 13 randomized controlled trials (RCTs) and one cohort study that included patients older than 18 years with TBI. These interventions included progesterone, EPO, and cyclosporine, with seven studies assessing progesterone. The general characteristics of the included studies are summarized in
Quality assessment
Overall, one included observational study had a good quality in NOS assessment.[
Outcomes
Mortality
A meta-analysis was conducted to compare the mortality rates between the intervention and control groups for TBI. Eleven studies[
We found that heterogeneity was minimized in the subgroup of EPO (I2 = 13%) and reduced among the progesterone group (I2 = 81%). Furthermore, we noticed that there was no statistically significant difference between the two groups in progesterone studies (OR = 0.74; 95% CI: 0.39–1.39, P = 0.35) and a statistically significant difference in the EPO intervention group (OR = 0.49; 95% CI: 0.31–0.78, P = 0.002) [
Efficacy of immunomodulators on neurological recovery
The neurological outcomes of TBI patients were evaluated in the included studies[
Figure 7:
Meta-analysis of the neurological outcomes of the patients after therapies. *Favorable Glasgow coma scale (GCS) refers to a score greater than 8, indicating a likelihood of good to moderate outcomes. **Unfavorable GCS means GCS indicates a score lower than 8, suggesting a higher risk of severe disabilities or poor prognosis. CI: Confidence interval.
Adverse events
Thromboembolic events
A meta-analysis of six studies[
Gastrointestinal tract disturbances
A meta-analysis of three[
We found that there is no statistically significant difference between the two groups; the OR was 1.11 (95% CI, 0.68–1.82, P = 0.68). There is minimal heterogeneity found among the included studies, with I2 = 15% [
Cardiac problems
A meta-analysis of five studies[
Sepsis
A meta-analysis of seven studies[
We found that there is no statistically significant difference between the two groups; the OR was 1.16 (95% CI, 0.84–1.60, P = 0.37). There is no heterogeneity found among the included studies, with I2 = 0% [
CNS disturbances
A meta-analysis of five studies[
Pneumonia
A meta-analysis of five studies[
DISCUSSION
TBI remains a significant public health concern worldwide, with substantial morbidity, mortality, and economic burden.[
The impact of EPO on TBI is a topic of significant interest and investigation within the medical research community. EPO, a hormone primarily known for its role in stimulating red blood cell production, has shown potential therapeutic benefits beyond its hematopoietic function, particularly in neuroprotection and neuroregeneration following TBI.[
Moreover, in our meta-analysis, progesterone did not show a significant improvement in mortality rates among the included research articles, as shown in
The neurological outcome was assessed through our meta-analysis. By collecting the GCS, we collected all the patients in the included studies who had a GCS of more than eight in one category, as this cut point reveals a good overall function of the patient. We found that many studies revealed a favorable outcome in patients managed by different immunomodulatory therapies. Many studies revealed similar results, as shown in recent meta-analyses.[
The adverse events of immunomodulatory therapies, including progesterone and EPO, have been the subject of investigation.[
CONCLUSION
While immunomodulatory therapies for TBI did not demonstrate a significant overall impact on mortality rates, subgroup analysis revealed promising outcomes with EPO intervention. In addition, these therapies did not significantly increase the risk of adverse events compared to conventional treatments. Future research should focus on further elucidating the efficacy of specific immunomodulatory agents and refining treatment protocols to optimize outcomes for TBI patients.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia. [Grant No. KFU241042].
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aloizos S, Evodia E, Gourgiotis S, Isaia EC, Seretis C, Baltopoulos GJ. Neuroprotective effects of erythropoietin in patients with severe closed brain injury. Turk Neurosurg. 2015. 25: 552-8
2. Bai XF, Gao YK. Recombinant human erythropoietin for treating severe traumatic brain injury. Medicine (Baltimore). 2018. 97: e9532
3. Bascones-Martinez A, Mattila R, Gomez-Font R, Meurman JH. Immunomodulatory drugs: Oral and systemic adverse effects. Med Oral Patol Oral Cir Bucal. 2014. 19: e24-31
4. Begemann M, Leon M, van der Horn HJ, van der Naalt J, Sommer I. Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: A meta-analysis. Sci Rep. 2020. 10: 16179
5. Bouras M, Asehnoune K, Roquilly A. Contribution of dendritic cell responses to sepsis-induced immunosuppression and to susceptibility to secondary pneumonia. Front Immunol. 2018. 9: 2590
6. Bouras M, Asehnoune K, Roquilly A. Immune modulation after traumatic brain injury. Front Med (Lausanne). 2022. 9: 995044
7. Cox CS, Hetz RA, Liao GP, Aertker BM, Ewing-Cobbs L, Juranek J. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017. 35: 1065-79
8. Donat CK, Scott G, Gentleman SM, Sastre M. Microglial activation in traumatic brain injury. Front Aging Neurosci. 2017. 9: 208
9. Goldstein FC, Caveney AF, Hertzberg VS, Silbergleit R, Yeatts SD, Palesch YY. Very early administration of progesterone does not improve neuropsychological outcomes in subjects with moderate to severe traumatic brain injury. J Neurotrauma. 2017. 34: 115-20
10. Hasan A, Deeb G, Rahal R, Atwi K, Mondello S, Marei HE. Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol. 2017. 8: 28
11. Hatton J, Rosbolt B, Empey P, Kryscio R, Young B. Dosing and safety of cyclosporine in patients with severe brain injury. J Neurosurg. 2008. 109: 699-707
12. Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013. 4: 30
13. Kalra S, Malik R, Singh G, Bhatia S, Al-Harrasi A, Mohan S. Pathogenesis and management of traumatic brain injury (TBI): Role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology. 2022. 30: 1153-66
14. Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019. 266: 2878-89
15. Konieczny MR, Senyurt H, Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013. 7: 3-9
16. Kroesen VM, Gröschel MI, Martinson N, Zumla A, Maeurer M, van der Werf TS. Non-Steroidal anti-inflammatory drugs as host-directed therapy for tuberculosis: A systematic review. Front Immunol. 2017. 8: 772
17. Lee J, Cho Y, Choi KS, Kim W, Jang BH, Shin H. Efficacy and safety of erythropoietin in patients with traumatic brain injury: A systematic review and meta-analysis. Am J Emerg Med. 2019. 37: 1101-7
18. Li ZM, Xiao YL, Zhu JX, Geng FY, Guo CJ, Chong ZL. Recombinant human erythropoietin improves functional recovery in patients with severe traumatic brain injury: A randomized, double blind and controlled clinical trial. Clin Neurol Neurosur. 2016. 150: 80-3
19. Lin C, He H, Li Z, Liu Y, Chao H, Ji J. Efficacy of progesterone for moderate to severe traumatic brain injury: A meta-analysis of randomized clinical trials. Sci Rep. 2015. 5: 13442
20. Liu L, Wei H, Chen F, Wang J, Dong JF, Zhang J. Endothelial progenitor cells correlate with clinical outcome of traumatic brain injury. Crit Care Med. 2011. 39: 1760-5
21. Liu M, Wang AJ, Chen Y, Zhao G, Jiang Z, Wang X. Efficacy and safety of erythropoietin for traumatic brain injury. BMC Neurol. 2020. 20: 399
22. Lo CK, Mertz D, Loeb M. Newcastle-ottawa scale: Comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014. 14: 45
23. Maas AI, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017. 16: 987-1048
24. Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y. Erythropoietin in traumatic brain injury (EPO-TBI): A double-blind randomised controlled trial. Lancet. 2015. 386: 2499-506
25. Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012. 6: 58
26. Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015. 157: 1683-96
27. Ritter M. Evidence-based pearls: Traumatic brain injury. Crit Care Nurs Clin North Am. 2023. 35: 171-8
28. Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014b. 312: 36-47
29. Said MF, Islam AA, Massi MN, Prihantono . Effect of erythropoietin administration on the expression of brain-derived neurotrophic factor, stromal cell-derived Factor-1, and neuron-specific enolase in traumatic brain injury: A literature review. Ann Med Surg (Lond). 2021. 69: 102666
30. Shakeri M, Boustani MR, Pak N, Panahi F, Salehpour F, Lotfinia I. Effect of progesterone ad-ministration on prognosis of patients with diffuse axonal injury due to severe head trauma. Clin Neurol Neurosurg. 2013. 115: 2019-22
31. Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD. A Clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014. 371: 2467-76
32. Soltani Z, Shahrokhi N, Karamouzian S, Khaksari M, Mofid B, Nakhaee N. Does progesterone improve outcome in diffuse axonal injury?. Brain Inj. 2017. 31: 16-23
33. Talving P, Lustenberger T, Kobayashi L, Inaba K, Barmparas G, Schnüriger B. Erythropoiesis stimulating agent administration improves survival after severe traumatic brain injury: A matched case control study. Annals of Surgery. 2010. 251: 1-4
34. Verboon LN, Patel HC, Greenhalgh AD. The immune system’s role in the consequences of mild traumatic brain injury (concussion). Front Immunol. 2021. 12: 620698
35. Weil ZM, Karelina K. Lifelong consequences of brain injuries during development: From risk to resilience. Front Neuroendocrinol. 2019. 55: 100793
36. Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007. 49: 391-402
37. Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014. 371: 2457-66
38. Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit Care. 2008. 12: R61
39. Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013. 10: 106
40. Zheng C, Gong J, Zang L, Song D, Ran X, Li J. Mechanism of progesterone in treatment of traumatic brain injury based on network pharmacology and molecular docking technology. Med Sci Monit. 2022. 28: e937564