- Department of Neurosurgery, Arkansas Neurosciences Institute, CHI St Vincent Infirmary, Little Rock, AR 72205, USA
- Department of Neurosurgery, Billings Clinic, Billings, MT 59101, USA
Correspondence Address:
Miguel Angel Lopez-Gonzalez
Department of Neurosurgery, Billings Clinic, Billings, MT 59101, USA
DOI:10.4103/2152-7806.181984
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lopez-Gonzalez MA, Dolan E. Endodermal cyst in pineal region: Rare location. Surg Neurol Int 06-May-2016;7:
How to cite this URL: Lopez-Gonzalez MA, Dolan E. Endodermal cyst in pineal region: Rare location. Surg Neurol Int 06-May-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/endodermal-cyst-in-pineal-region-rare-location/
Abstract
Background:Pineal tumors are very uncommon intracranial lesions, and endodermal cysts in this location are extremely rare.
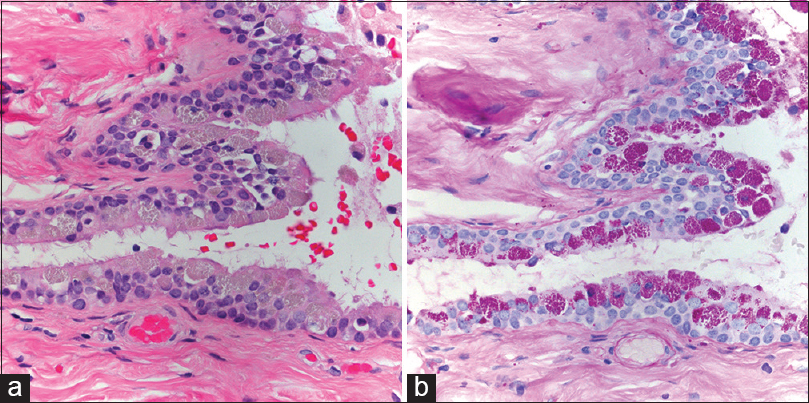
Case Description:A 49-year-old right-handed female presented with 3 weeks history of progressive dizziness and imbalance. Imaging studies showed 1.8 cm × 1.7 cm × 1.8 cm pineal lesion with small enhancing mural component displacing ventrally the quadrigeminal plate and narrowing of aqueduct of Sylvius without hydrocephalus. In addition, she was found with small interhemispheric lipoma, and small posterior falx possible meningioma. Cerebrospinal fluid markers obtained by lumbar puncture were all negative. She underwent tumor resection, and final pathology reported endodermal cyst. No new deficits were encountered, and her gait imbalance improved significantly by 3 months follow-up.
Conclusions:With evidence of enlargement or symptomatic pineal lesions, surgical consideration is necessary. Among pineal lesions, endodermal cysts are extremely uncommon and although benign pathology, long-term follow-up is advised due to unknown chronic behavior.
Keywords: Endodermal cyst, pineal region, pineal tumor
INTRODUCTION
Pineal region tumors are very uncommon, accounting for <1% of adult brain tumors. To the best of our knowledge, among the multiple lesions in the differential diagnosis, endodermal cyst has not been reported only within the pineal region.[
CASE DESCRIPTION
Case presentation
A 49-year-old right-handed female, chronic and heavy smoker, with high blood pressure who was admitted to medicine service with 3 weeks of progressive dizziness and gait imbalance confirmed on physical examination. Rest of her neurological examination was intact. A lumbar puncture was obtained, and then she was managed with a low dose of steroids, and acetazolamide for 2 weeks until obtaining cerebrospinal fluid (CSF) results. She continued to be symptomatic, and CSF results showed chorionic gonad beta-Human Chorionic Gonadotropin (bHCG), carcinoembryonic antigen, and alpha-fetoprotein within normal limits.
Imaging findings
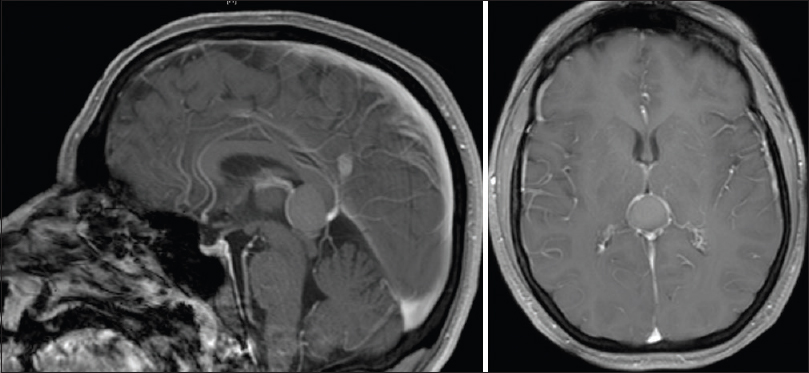
The head computed tomography (CT) scan showed hyperdense pineal lesion. The brain magnetic resonance imaging (MRI) showed a partially cystic pineal region mass of 1.8 cm of major diameter containing T1 hyperintense proteinaceous debris, and T1 isointense/T2 hyperintense enhancing mural nodule within the right lateral aspect of the lesion, no restricted diffusion, and increased signal on fluid-attenuated inversion recovery (FLAIR). The lesion splayed the internal cerebral veins laterally, and displaced tectum and superior colliculi inferiorly, producing a narrowing of aqueduct of Sylvius [
Surgical procedure
Surgery was recommended due to worsening of gait imbalance, lack of improvement with steroids and acetazolamide, relatively large size of the lesion with mass effect on midbrain, and for diagnosis due to atypical imaging features. A right frontal external ventricular drain was placed in anticipation of possible postoperative hydrocephalus. The patient was placed in a semi-sitting position and midline suboccipital craniotomy was performed using burr holes above the transverse sinus exposing torcula. The dura was opened in a Y fashion, and 4-0 nylon suture were placed to retract transverse sinus and torcula superiorly. The favorable tentorial angle facilitated the supracerebellar infratentorial approach, using microsurgical techniques, stereotactic navigation and electrophysiology monitoring (somatosensory evoked potentials and brainstem auditory evoked potentials). The capsule of the tumor was solid, yellowish, and brown with thick and yellow gel-like internal component. The capsule was subtotally resected, leaving a small segment with tight adhesions to the right internal cerebral vein. After lesion resection, the anterior and superior aspect of the third ventricle was visualized. The dura was closed in a watertight fashion with 5-0 prolene and surgical glue using the surgical microscope.
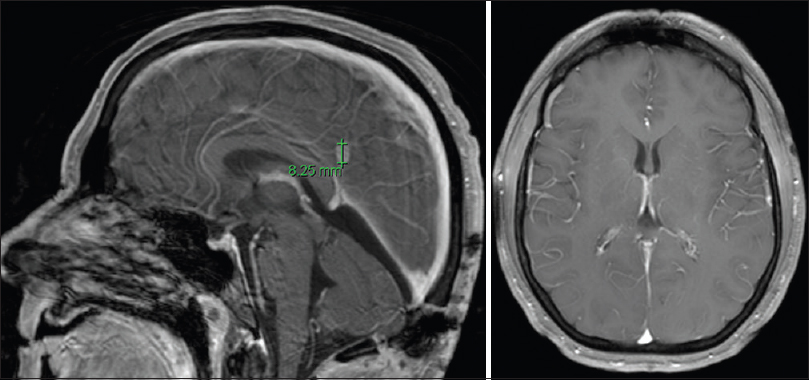
The postoperative MRI showed gross total resection [
The ventricular drain was kept clamped without any signs of hydrocephalus on postoperative images, removed on postoperative day 2, and discharged home on a postoperative day 4. No new deficits were encountered, and her gait imbalance improved significantly by 3 months follow-up.
DISCUSSION
The preoperative imaging findings in this patient as described above are not specific, and atypical for conventional cystic lesions in this location due to the enhancing mural component. The typical endodermal cyst shows hyperdensity on the head CT scan, as well as mixed iso- and hyper-intensity on T1 and T2 MRI, which are considered caused by high content of proteinaceous debris. FLAIR images show hyperintensity, and mild or no restriction on diffusion weighted images, which differs from significantly restricted diffusion of epidermoid cysts. There is usually no contrast enhancement on endodermal cysts which differs from our present case. In general, lack of contrast enhancement of the cyst wall, and of bone destruction is useful for distinguishing endodermal cysts from other cystic lesions such as schwannomas or cystic meningiomas.[
CONCLUSIONS
Endodermal cyst is another differential diagnosis that should be considered within the pineal region. The ideal treatment of symptomatic endodermal cyst is a total resection of the lesion, including all cyst contents to decrease the possibility of chemical meningitis, as well as the complete capsule resection to avoid the risk of recurrence. In locations where the capsule is densely adhered to important neurovascular structures, a partial resection is reasonable. Long-term follow-up is advised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We appreciate neuropathology evaluation and images by Dr. Caterina Gianini, Dr. Ronald Linfesty, and Dr. Jeffrey Smith. Also, appreciate Sharon Partington for the review of manuscript.
References
1. Batuk D, Koichiro Y, Tsukasea K, Tetsuo K, Ando M. Cerebellopontine angle endodermal cyst: A rare occurrence. Neurol India. 2010. 58: 676-7
2. Bejjani GK, Wright DC, Schessel D, Sekhar LN. Endodermal cysts of the posterior fossa.Report of three cases and review of the literature. J Neurosurg. 1998. 89: 326-35
3. Cheng JS, Cusick JF, Ho KC, Ulmer JL. Lateral supratentorial endodermal cyst: Case report and review of literature. Neurosurgery. 2002. 51: 493-9
4. Choi JE, Seol HJ, Cho YS. Giant petroclival endodermal cyst with xanthogranulomatous changes. J Neurosurg Pediatr. 2013. 12: 284-7
5. Goel A, Muzumdar D, Chagla A. Endodermal cyst anterior and anterolateral to the brainstem: A report of an experience with seven cases. Br J Neurosurg. 2005. 19: 163-6
6. Graziani N, Dufour H, Figarella-Branger D, Donnet A, Bouillot P, Grisoli F. Do the suprasellar neurenteric cyst, the Rathke cleft cyst and the colloid cyst constitute a same entity?. Acta Neurochir (Wien). 1995. 133: 174-80
7. Hasegawa T, Kondziolka D, Hadjipanayis CG, Flickinger JC, Lunsford LD. The role of radiosurgery for the treatment of pineal parenchymal tumors. Neurosurgery. 2002. 51: 880-9
8. Konovalov AN, Pitskhelauri DI. Principles of treatment of the pineal region tumors. Surg Neurol. 2003. 59: 250-68
9. Nakajima M, Miyajima M, Hishii M, Arai H, Sato K, Fujii H. Endodermal cyst of the quadrigeminal cistern: Case report. Surg Neurol. 2001. 56: 385-8
10. Regis J, Bouillot P, Rouby-Volot F, Figarella-Branger D, Dufour H, Peragut JC. Pineal region tumors and the role of stereotactic biopsy: Review of the mortality, morbidity, and diagnostic rates in 370 cases. Neurosurgery. 1996. 39: 907-12
11. Teufack S, Campbell P, Moshel Y. Intracranial neurenteric cysts: Two atypical cases and review of the literature. Jefferson Hosp Neurosci J. 2011. 6: 18-21