- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Department of Otorhinolaryngology Head and Neck Surgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, Fukuoka, Japan.
- Department of Health Sciences, Graduate School of Medical Sciences, Kyushu University,Fukuoka, Japan.
- Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University,Fukuoka, Japan.
- Department of Neurosurgery, Harasanshin Hospital, Fukuoka, Japan.
Correspondence Address:
Nobutaka Mukae, Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
DOI:10.25259/SNI_542_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobutaka Mukae1, Daisuke Kuga1, Daisuke Murakami2, Noritaka Komune2, Yusuke Miyamoto2, Takafumi Shimogawa1, Ayumi Sakata3, Hiroshi Shigeto4, Toru Iwaki5, Takato Morioka6, Masahiro Mizoguchi1. Endonasal endoscopic surgery for temporal lobe epilepsy associated with sphenoidal encephalocele. 27-Jul-2021;12:379
How to cite this URL: Nobutaka Mukae1, Daisuke Kuga1, Daisuke Murakami2, Noritaka Komune2, Yusuke Miyamoto2, Takafumi Shimogawa1, Ayumi Sakata3, Hiroshi Shigeto4, Toru Iwaki5, Takato Morioka6, Masahiro Mizoguchi1. Endonasal endoscopic surgery for temporal lobe epilepsy associated with sphenoidal encephalocele. 27-Jul-2021;12:379. Available from: https://surgicalneurologyint.com/surgicalint-articles/10991/
Abstract
Background: Temporal lobe epilepsy (TLE) associated with temporal lobe encephalocele is rare, and the precise epileptogenic mechanisms and surgical strategies for such cases are still unknown. Although the previous studies have reported good seizure outcomes following chronic subdural electrode recording through invasive craniotomy, only few studies have reported successful epilepsy surgery through endoscopic endonasal lesionectomy.
Case Description: An 18-year-old man developed generalized convulsions at the age of 15 years. Despite treatment with optimal doses of antiepileptic drugs, episodes of speech and reading difficulties were observed 2–3 times per week. Long-term video electroencephalogram (EEG) revealed ictal activities starting from the left anterior temporal region. Magnetic resonance imaging revealed a temporal lobe encephalocele in the left lateral fossa of the sphenoidal sinus (sphenoidal encephalocele). Through the endoscopic endonasal approach, the tip of the encephalocele was exposed. A depth electrode was inserted into the encephalocele, which showed frequent spikes superimposed with high-frequency oscillations (HFOs) suggesting intrinsic epileptogenicity. The encephalocele was resected 8 mm from the tip. Twelve months postoperatively, the patient had no recurrence of seizures on tapering of the medication.
Conclusion: TLE associated with sphenoidal encephalocele could be controlled with endoscopic endonasal lesionectomy, after confirming the high epileptogenicity with analysis of HFOs of intraoperative EEG recorded using an intralesional depth electrode.
Keywords: Depth electrode, Endoscopic endonasal surgery, Epileptogenicity, Sphenoidal encephalocele, Temporal lobe encephalocele
INTRODUCTION
Temporal encephaloceles are rare presentation,[
CASE DESCRIPTION
An 18-year-old right-handed man was referred to our department for surgical treatment of drug-resistant epilepsy. He had no history of head trauma. He developed generalized seizures at age 15 years. Following treatment with optimal dosage of antiepileptic drugs, including sodium valproate, zonisamide, lacosamide, and clobazam, his generalized seizures were controlled; however, episodes of speech and reading difficulties were observed 2–3 times a week.
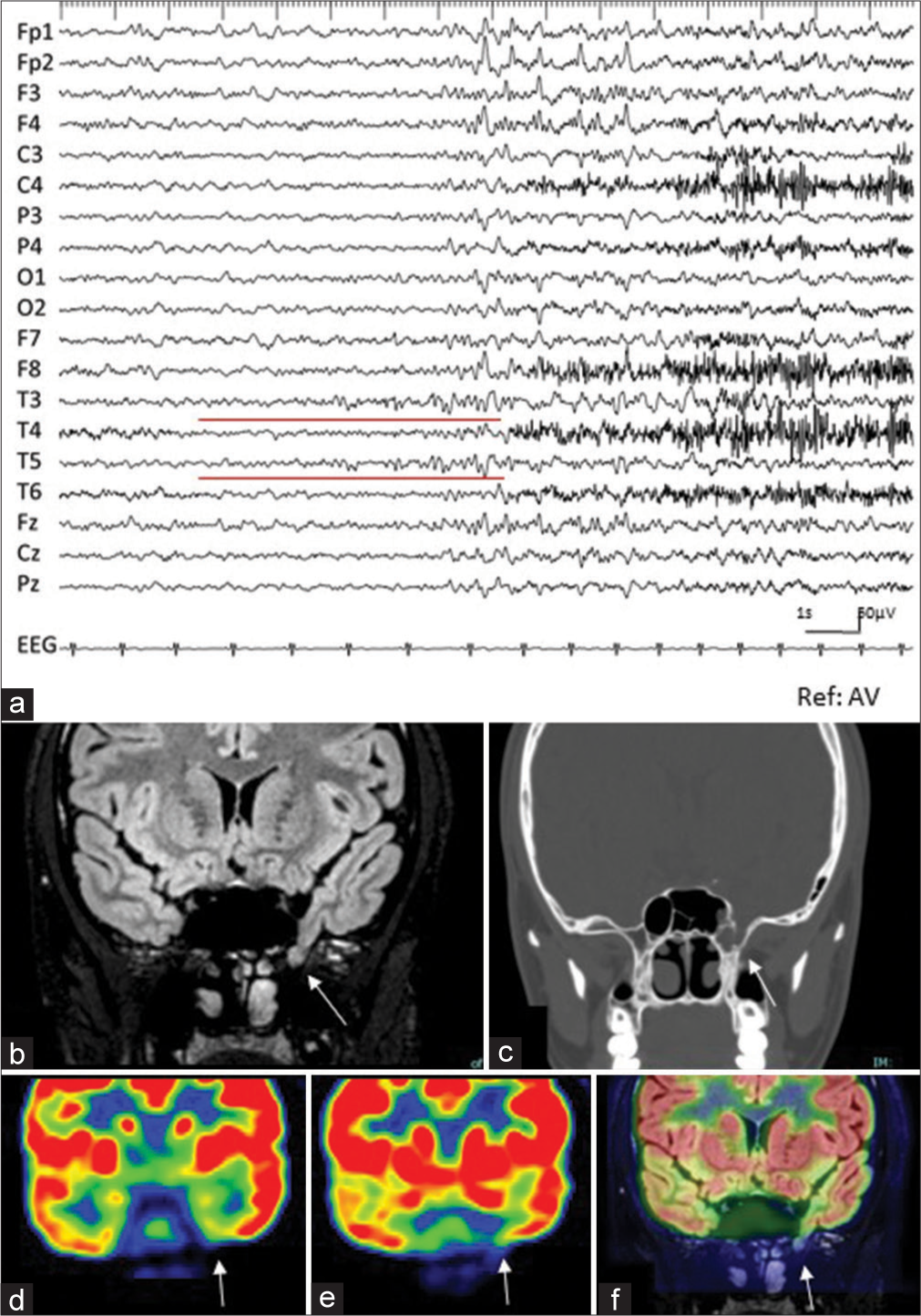
The patient was considered normal neurologically. Long-term video electroencephalogram (EEG) demonstrated ictal discharges with low-amplitude, rhythmic, irregular α waves appearing in T3 and T5; these waves demonstrated increasing amplitude, with frequency changing from δ to δ, when the patient experienced a dull sensation that lasted several seconds [
Figure 1:
(a) Preoperative electroencephalogram recorded at the start of the habitual seizure (sensitivity: 10 µV, time constant: 0.1 s, high cut filter: 30 Hz, reference: average). Ictal activities begin with rhythmic alpha activity at T3 and T5 (red line). (b) Coronal view of preoperative magnetic resonance image (MRI) with fluid-attenuated inversion recovery (FLAIR) sequence demonstrates that protrusion of a part of the left temporal lobe is confirmed in the lateral fossa of the sphenoid sinus, indicating sphenoidal encephalocele (Red arrow). (c) Coronal computed tomography (CT) at a comparable level with (b) confirms the bone defect on the left lateral wall of the sphenoid sinus (white arrow). (d and e) Coronal view of 18-F fluorodeoxyglucose (FDG)-positron emission tomography (PET) image at the hippocampus (d) and a few centimeters behind the sphenoidal encephalocele and at the sphenoidal encephalocele (e). Decreased accumulation of FDG is observed around the left medial and basal temporal area (white arrows). (f) The fusion image of FDG-PET (e) and FLAIR-MRI (b) clearly shows that markedly decreased accumulation of FDG is observed at the tip of the encephalocele (white arrow).
Magnetic resonance imaging (MRI) revealed a protrusion of the left medial temporal lobe from the middle cranial fossa to the lateral fossa of the sphenoid sinus [
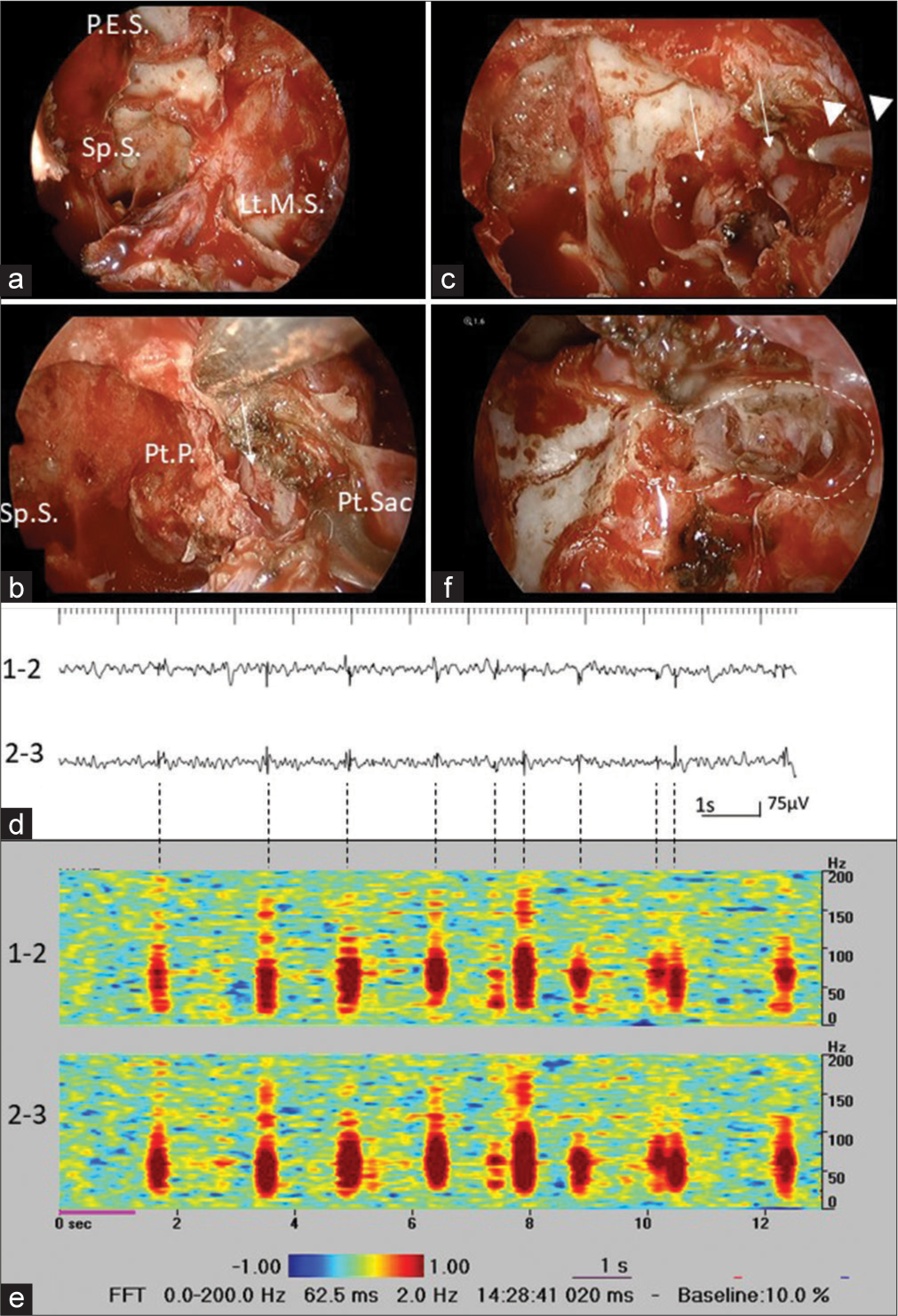
Endoscopic endonasal removal of the sphenoidal encephalocele was performed. After opening the sphenoid sinus, posterior ethmoid sinus, and left maxillary sinus from both the nasal cavities, the posterior wall of the left maxillary sinus was drilled, and the pterygopalatine sac was exposed [
Figure 2:
(a) Posterior wall of the left maxillary sinus is drilled, and the pterygopalatine sacs are exposed, after opening the sphenoid sinus (Sp.S), posterior ethmoid sinus (P.E.S.), and left maxillary sinus (Lt.M.S.) from both nasal cavities. (b) While flipping the pterygopalatine sac (Pt. Sac) outwards, the base of the pterygoid plate (Pt.P.) and the sphenoid sinus septum connected to it is being removed, and encephalocele is confirmed (white arrow). (c) A depth electrode (white arrow heads) is inserted into the encephalocele (white arrows). (d) Intraoperative EEG reveals frequent spikes in the encephalocele (bipolar recording, sensitivity: 20 μV, time constant: 0.1 s, high cut filter: 60 Hz). (e) Time-frequency analysis reveals high-frequency oscillations ranging from 50 Hz to 150 Hz, superimposing to the spikes. (f) The tip of encephalocele is successfully resected (in the white dotted line).
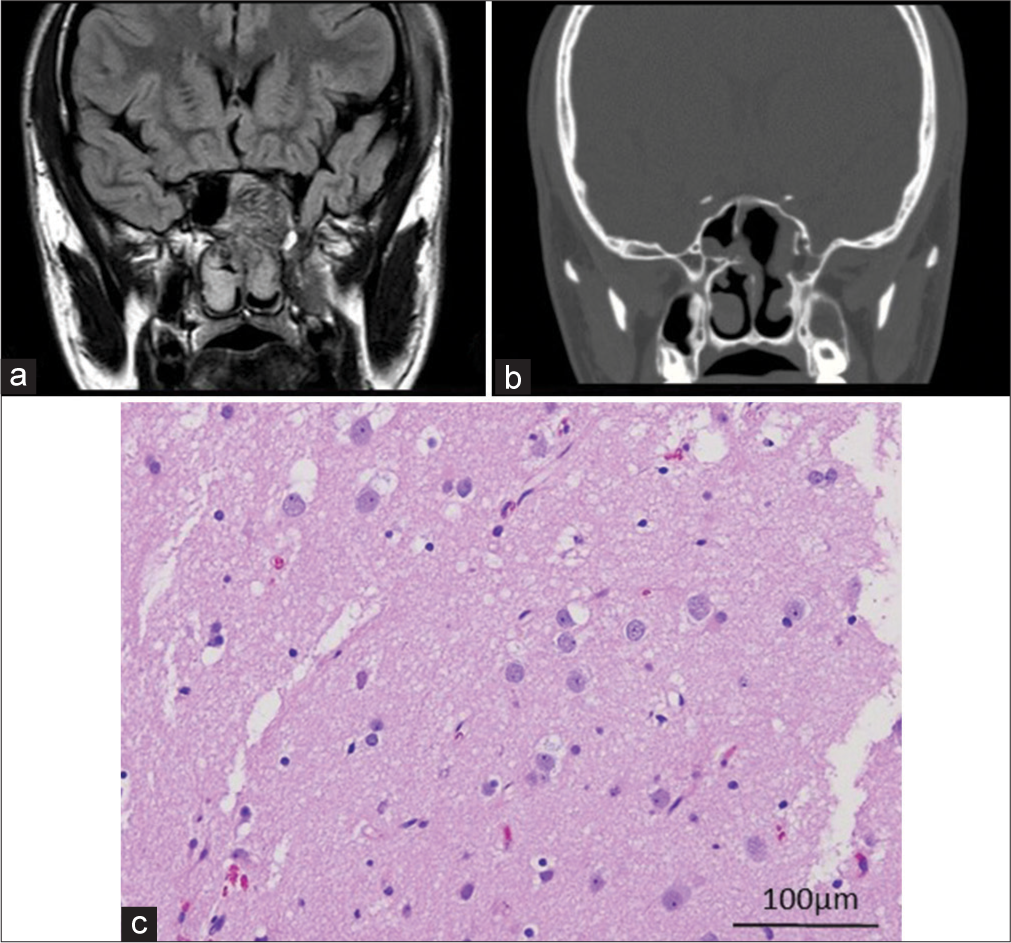
The patient’s postoperative course was uneventful, without leakage of CSF. Postoperative MRI confirmed that the sphenoidal encephalocele was resected 8 mm from the tip [
DISCUSSION
Epileptiform discharges around the cortical area of the encephalocele have been previously recorded with intraoperative or chronic electrocorticography using subdural grid electrodes inserted through invasive craniotomy.[
Due to the recent development in endoscopy, there are increasing surgical reports of temporal encephalocele resection using the endoscopic endonasal approach.[
The drawback of the endoscopic approach was that en bloc resection of the encephalocele could not be performed. Because of the small specimens obtained with piecemeal resection in the present case, it was difficult to show the structural abnormality of the cortical lamination. Neurons appeared largely normal, but several reactive astrocytes were observed. Panov et al.[
CONCLUSION
Although our observations reported herein were derived from a single case, temporal lobe encephalocele-related TLE could be controlled with endoscopic endonasal lesionectomy. This involves detecting the high epileptogenicity of the encephalocele by analysis of HFOs of the intraoperative EEG recorded with intralesional depth electrode.
Statement of ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from the patient’s parents for publication of this case report and any accompanying images.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Abou-Hamden A, Lau M, Fabinyi G, Berkovic S, Jackson GD, Mitchell LA. Small temporal pole encephaloceles: A treatable cause of “lesion negative” temporal lobe epilepsy. Epilepsia. 2010. 51: 2199-202
2. Bozkurt G, Turri-Zanoni M, Coden E, Russo F, Elhassan HA, Gallo S. Endoscopic endonasal transpterygoid approach to sphenoid sinus lateral recess defects. J Neurol Surg B Skull Base. 2020. 81: 553-61
3. Byrne RW, Smith AP, Roh D, Kanner A. Occult middle fossa encephaloceles in patients with temporal lobe epilepsy. World Neurosurg. 2010. 73: 541-6
4. Cho JR, Joo EY, Koo DL, Hong SC, Hong SB. Clinical utility of interictal high-frequency oscillations recorded with subdural macroelectrodes in partial epilepsy. J Clin Neurol. 2012. 8: 22-34
5. El-Tarabishi MN, Fawaz SA, Sabri SM, El-Sharnobi MM, Sweed A. A modification of endoscopic endonasal approach for management of encephaloceles in sphenoid sinus lateral recess. Eur Arch Otorhinolaryngol. 2016. 273: 4305-14
6. Faulkner HJ, Sandeman DR, Love S, Likeman M, Nunez DA, Lhatoo SD. Epilepsy surgery for refractory epilepsy due to encephalocele: A case report and review of the literature. Epileptic Disord. 2010. 12: 160-6
7. Kikuchi K, Togao O, Yamashita K, Momosaka D, Nakayama T, Kitamura Y. Diagnostic accuracy for the epileptogenic zone detection in focal epilepsy could be higher in FDG-PET/ MRI than in FDG-PET/CT. Eur Radiol. 2021. 31: 2915-22
8. Lai SY, Kennedy DW, Bolger WE. Sphenoid encephaloceles: Disease management and identification of lesions within the lateral recess of the sphenoid sinus. Laryngoscope. 2002. 112: 1800-5
9. Landreneau FE, Mickey B, Coimbra C. Surgical treatment of cerebrospinal fluid fistulae involving lateral extension of the sphenoid sinus. Neurosurgery. 1998. 42: 1101-5
10. Mukae N, Morioka T, Torio M, Sakai Y, Shimogawa T, Sakata A. Periodic discharges with high frequency oscillations recorded from a cerebellar gangliocytoma in an epileptic infant. Surg Neurol Int. 2021. 12: 98
11. Olasunkanmi A, Fisher T, Shin H, Zanation A, Sasaki-Adams D. Endoscopic endonasal approach for resection of middle fossa encephalocele for treatment of refractory temporal lobe epilepsy. J Neurol Surg B Skull Base. 2016. 77: P046
12. Panov F, Li Y, Chang EF, Knowlton R, Cornes SB. Epilepsy with temporal encephalocele: Characteristics of electrocorticography and surgical outcome. Epilepsia. 2016. 57: e33-8
13. Wilkins RH, Radtke RA, Burger PC. Spontaneous temporal encephalocele. Case report. J Neurosurg. 1993. 78: 492-8
14. Wind JJ, Caputy AJ, Roberti F. Spontaneous encephaloceles of the temporal lobe. Neurosurg Focus. 2008. 25: E11
15. Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004. 127: 1496-506