- Department of Neurosurgery, Faculty of Medicine, Tanta University, Tanta, Gharbia, Egypt.
DOI:10.25259/SNI_588_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sherif Elsayed Elkheshin, Ahmed Y. Soliman. Endoscopic interlaminar lumbar discectomy: How to decrease the learning curve. 18-Nov-2020;11:401
How to cite this URL: Sherif Elsayed Elkheshin, Ahmed Y. Soliman. Endoscopic interlaminar lumbar discectomy: How to decrease the learning curve. 18-Nov-2020;11:401. Available from: https://surgicalneurologyint.com/surgicalint-articles/10404/
Abstract
Background: Herniated lumbar disc is a common cause of lumbosacral pain. Endoscopic interlaminar lumbar discectomy (ILD) is a well-established technique that provided comparable results to micro-discectomy. The aim of the study is to describe the learning curve of endoscopic ILD and explore measures that could improve effectiveness and decrease blood loss and operative time with accumulation of reasonable experience.
Methods: This retrospective cohort study included 65 patients presenting with symptomatic herniated lumbar disc who underwent endoscopic ILD. Patients were divided into two groups: Group I (standard technique) and Group II (modified technique). Collected data included patients’ age, gender, preoperative manifestations, visual analog score (VAS) for pain, Oswestry Disability Index (ODI), disc level, operative time, intraoperative blood loss, complications, and follow-up data at 1, 6, and 12 months postoperatively. Primary outcomes included total operative time, amount of intraoperative blood loss, and post-operative improvement in pain. Secondary outcomes included intraoperative complications, rate of conversion to open surgery, and recurrence.
Results: Post-operative VAS and ODI improved significantly in both groups. Mean total surgical time and intraoperative blood loss were significantly lower in Group II compared to Group I (P
Conclusion: The learning curve of endoscopic ILD was shallow with standard technique, indicating difficulties in mastering the procedure. The proposed modified technique helped reaching the required level of proficiency in the early phase of the curve, providing a significant reduction in operative time and blood loss, with comparable effectiveness and safety as the standard technique.
Keywords: Disc herniation, Interlaminar discectomy, Learning curve, Neuroendoscopy
INTRODUCTION
Disc prolapse of the lumbosacral region is one of the most commonly encountered conditions in neurosurgery. Surgical management largely contributes to alleviation of symptoms in selected indicated patients.[
The introduction of endoscopy into surgical fields provided a means for less invasive techniques, allowing better visualization of the surgical field, smaller incisions, paraspinal muscle splitting, and shorter hospital stays.[
Although microscopic discectomy is still the gold standard technique for surgical management of disc prolapse, endoscopic techniques are promising alternatives that are increasingly used and provided comparable results to microsurgery.[
During an early phase of learning curve, improvement in performance is observed with increased number of performed surgeries. A plateau phase then follows where the procedure is well mastered by the surgeon and higher number of performed cases will result in minimal change in quality of performance. The number required to reach the plateau phase differs from one procedure to another, and it is affected by the surgeon’s prior experience in similar techniques.[
Despite being a well-established technique, few studies assessed the learning curve of ILD, and most of them provided controversial reports. The present study was conducted to describe the learning curve of endoscopic ILD and to explore measures that could improve effectiveness and decrease both blood loss and operative time with the progress of experience.
MATERIALS AND METHODS
Study design, settings, and ethical considerations
This retrospective cohort study was conducted on 65 patients presenting with symptomatic herniated lumbar disc and operated through endoscopic inter laminar approach, at Tanta University Hospitals during the period from October 1, 2015, to November 30, 2018. Ethical approval was obtained from the Institutional Review Board, Faculty of Medicine, Tanta University. Confidentiality of the patients’ data was maintained by assigning code numbers to patients that were known only by the researchers.
Eligibility criteria
The present study included patients with symptomatic herniated lumbar disc for whom endoscopic ILD was indicated. The indication for surgery was based on radicular pain response and/or existing neurologic deficits. Magnetic resonance imaging 1.5 Tesla was obtained for all patients.
Data collection
The patients’ data were thoroughly reviewed. The collected data included patients’ age, sex, presenting manifestations, preoperative levels of visual analog score (VAS) for pain, and Oswestry Disability Index (ODI) as well as level of the herniated lumbar disc. In addition, operative details, including total operative time, the amount of intraoperative blood loss, and complications, were recorded. Data of follow-up at 1, 6, and 12 months postoperatively were collected as regard VAS, ODI, and symptomatic recurrence.
Surgical technique
In the current study, patients were divided into two groups according to the chronological order of the surgery. Group I was operated upon using the standard technique of endoscopic ILD, while a modified technique was used in Group II.
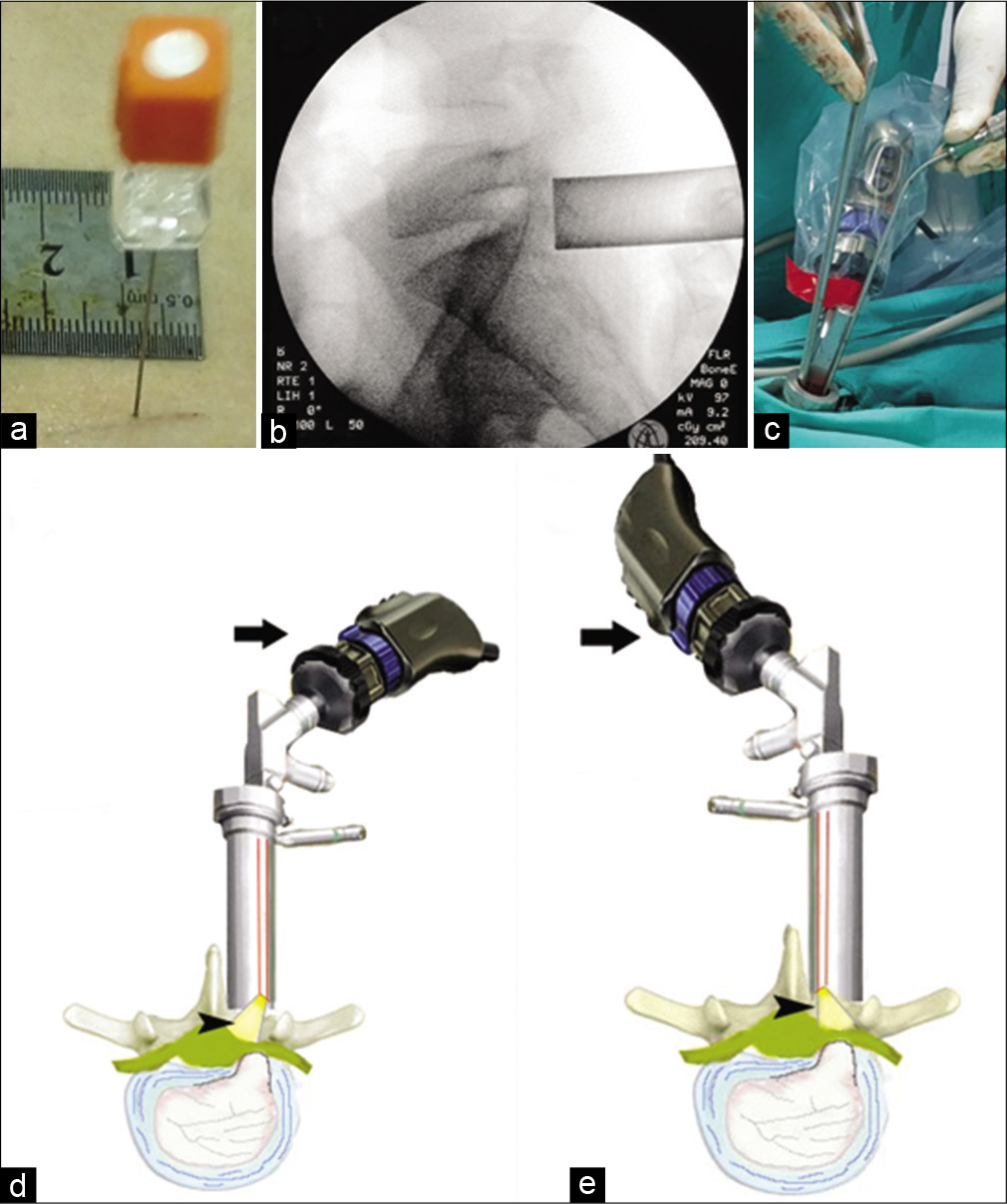
All patients were operated on using the EasyGo® endoscopic system (Karl Storz company, Tuttlingen, Germany) under general anesthesia with local lidocaine infiltration. All patients in Group I were positioned prone on Wilson frame. After initial sterilization of the skin, a spinal needle was inserted in place to confirm the level under fluoroscopic guidance [
Figure 1:
(a) Spinal needle is inserted for fluoroscopic level verification. (b) Intraoperative fluoroscopy confirming proper sheath location following dilatation. (c) The usual surgeon position ipsilateral to left level disc with an indispensable need for extra-long instruments. (d) A diagram showing the ipsilateral camera-lens complex yields good illumination (black arrowhead) but not the best for a far lateral disc. (e) An illustration showing the contralateral camera lens complex yields superior light (black arrowhead) and a better view for the extreme lateral disc herniation. Note the camera head is rotated up 180 degrees to match the surgeon side field (black arrow). Some camera firmware can rotate the image on display without rotation of the camera body.
The technique time-consuming steps were: Necessity to rongeur more from lamina to obtain full view of the nerve displaced by the offending disc, troublesome epidural bleeding requiring frequent suctioning, and necessity to introduce the least instrument set to keep the working channel less crowded. In Group II, these issues were overcome by (1) keeping the patient at knee-chest position with hip flexed to widen the interlaminar space and posterior disc height; (2) intravenous infusion of the anesthetic medication dexmedetomidine HCL (Precedex ®), an α2-adrenergic receptor agonist with a clinically proven effect (26) in reduction of intraoperative bleeding, (aiming to reduce epidural bleeding); and (3) rotation of the lenscope 180 degrees in the working sheath to obtain both better view of the nerve root and more space for surgical instruments [
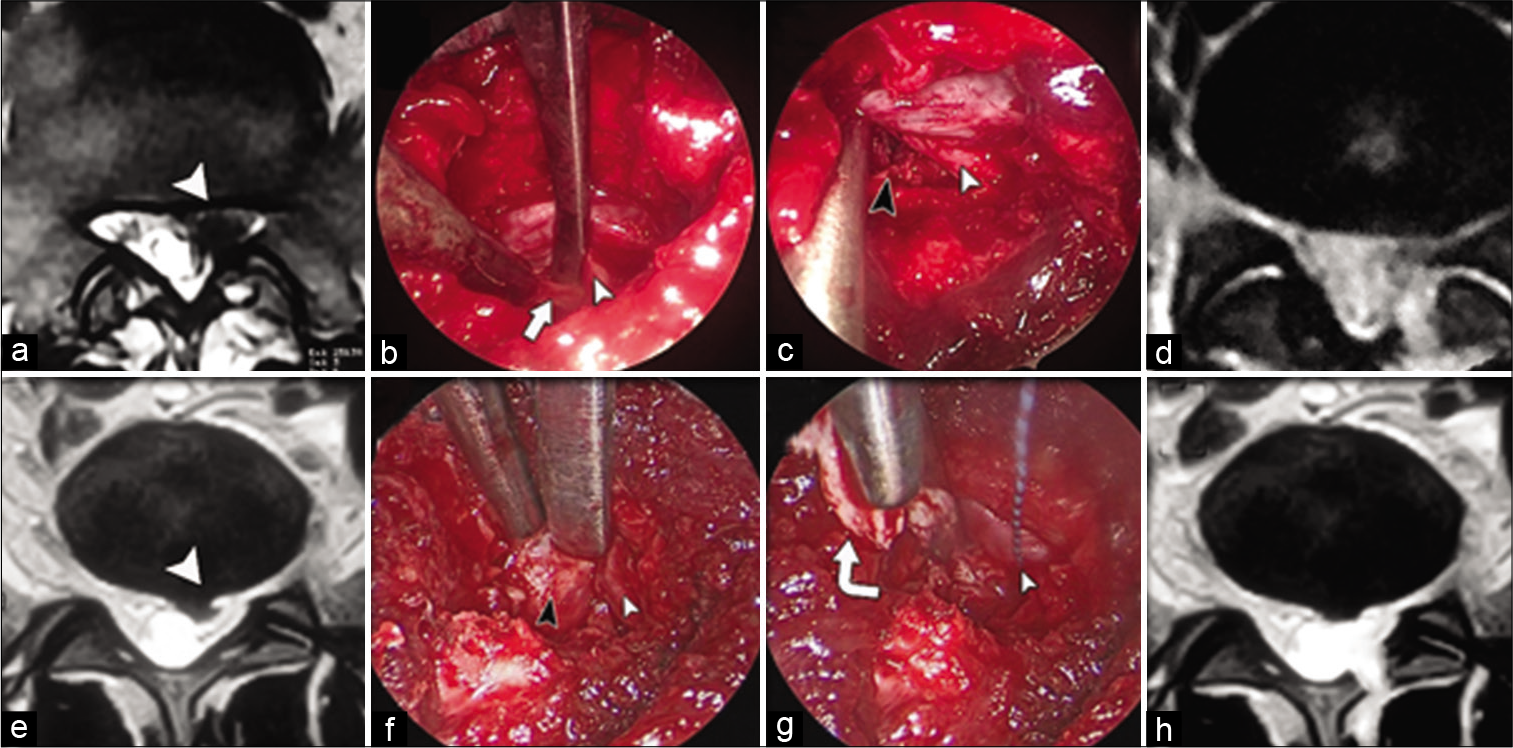
Drilling of the lower part of upper lamina was done to fashion a keyhole corridor to access the root and corresponding disc. Ligamentum flavum was resected to reveal the nerve root that was retracted medially, and disc removal with rongeur was accomplished by standard methods [
Figure 2:
Same patient (a) pre-operative axial MRI showing left L5S1 herniated disc. (b) Intraoperative image with endoscope sheath position as shown in Figure 1d, the dissector is gently retracting the S1 root (white arrowhead) and passed underneath the remaining part of ligamentum flavum (white arrow). (c) Camera-lens complex in contralateral position [as shown in Figure 1e] showing full root path (white arrowhead) after annular incision and removal of the disc (black arrowhead). (d) Postoperative MRI is revealing adequate disc removal. Images are for another patient. (e) Axial MRI is showing L4-5 left herniated disc. (f) The L5 nerve root (white arrow) is retracted to reveal subradicular disc herniation (black arrow). (g) The nerve root (white arrow) is kept in retraction by cottonoid, followed by annulotomy and removal of the herniated disc by rongeur (curved white arrow). (h) Post-operative MRI is confirming the success of the procedure.
Outcomes
The primary studied outcomes included total operative time, amount of intraoperative blood loss, post-operative pain decline, and post-operative MRI [
Statistical analysis
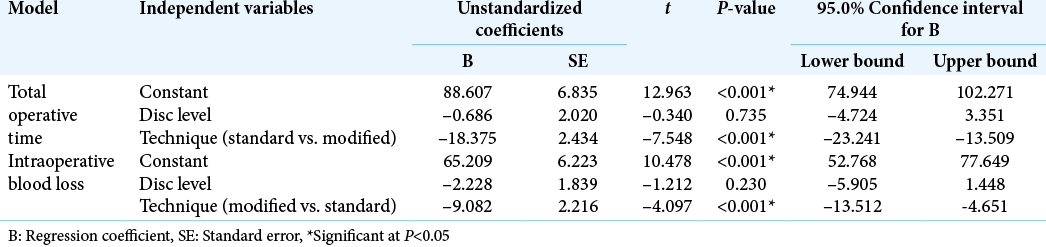
Data were analyzed using the Statistical Package for the Social Sciences software, version 26.0. Qualitative variables were represented as number and percentage and associations were tested using Pearson’s Chi-square test for independence, Fisher’s exact test, or Fisher-Freeman-Halton exact test as appropriate. All quantitative variables followed normal distribution. They were summarized as means and standard deviations, and differences between the groups were assessed using independent samples t-test. Pearson’s correlation was conducted to examine relationship between experience and outcome measures. Multiple regression analysis was conducted to examine the effects of the disc level and the surgical technique on the total surgical time and intraoperative blood loss. P < 0.05 was adopted for significant results.
RESULTS
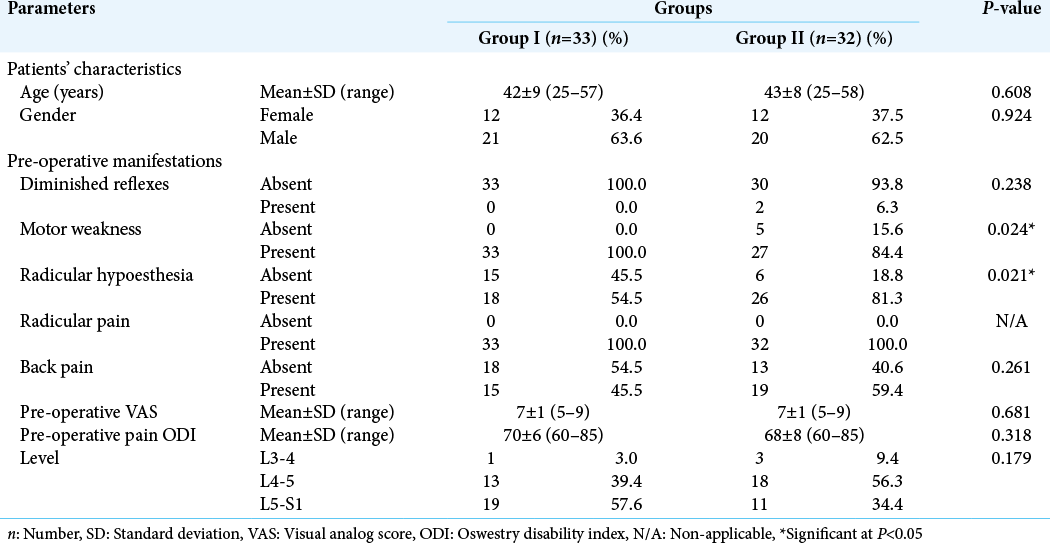
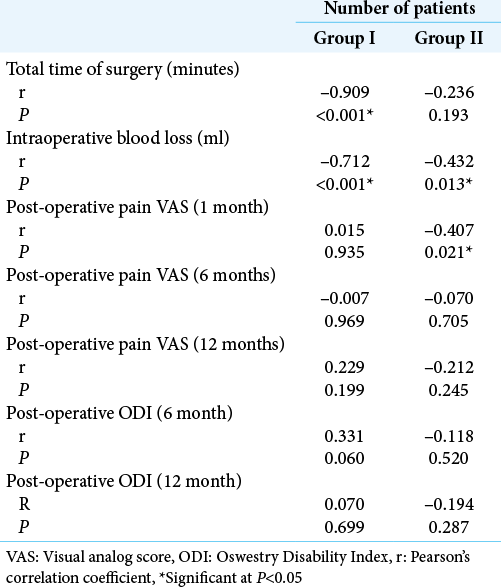
The mean age was 42 ± 9 years in Group I and 43 ± 8 years in Group II with no significant difference (P = 0.608). In both groups, male patients outnumbered the female patients (63.6% vs. 36.4% in Group I; and 62.5% vs. 37.5% in Group II), with no significant difference (P = 0.924). Motor weakness and radicular pain were the most common manifestations in both groups. Group I had significantly higher percentage of patients with motor weakness than Group II (100% vs. 84.4%, respectively; P = 0.024). On the other hand, Group II had a significantly higher percentage of patients with radicular hypoesthesia (81.3% vs. 54.5%, respectively; P = 0.021). The mean preoperative VAS was similar in the two groups (VAS = 7 ± 1, P = 0.681). There was no significant difference in pre-operative pain ODI between the two groups (P = 0.318). Nearly half the cases in Group I had disc prolapse at L5-S1 level, while nearly half the cases in Group II had disc prolapse at L4-5 level, with no significant difference (P = 0.179) [
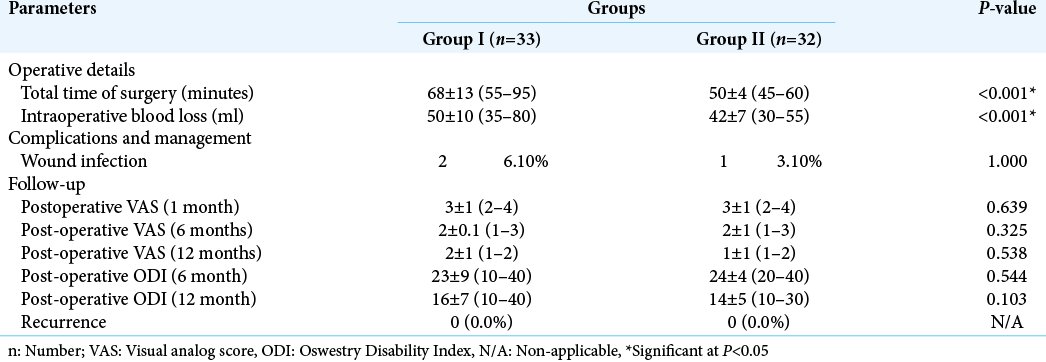
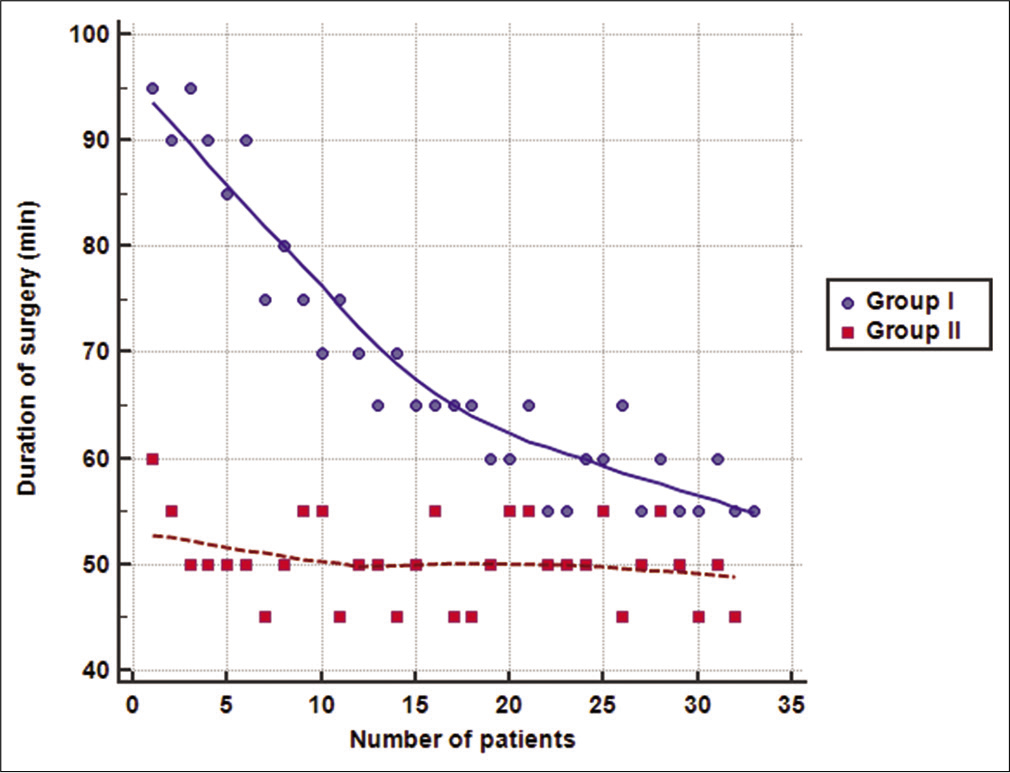
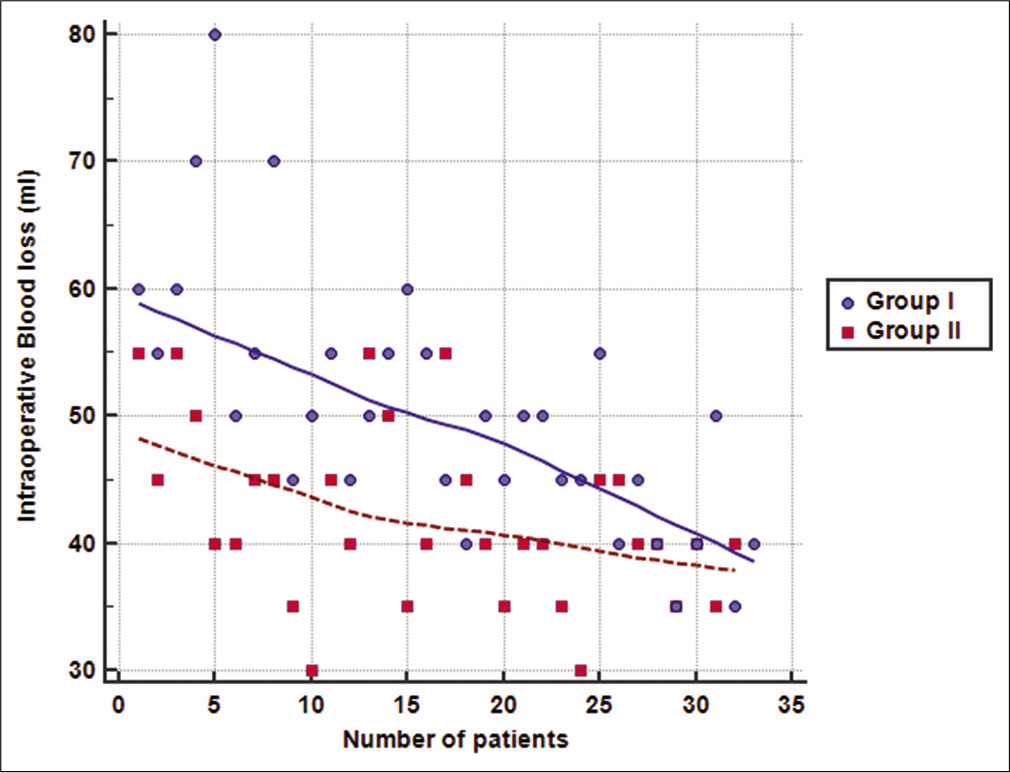
The mean total operative time was significantly lower in Group II compared to Group I (50 ± 4 vs. 68 ± 13 min, respectively; P < 0.001). Moreover, the mean amount of intraoperative blood loss was also significantly lower in Group II compared to Group I (42 ± 7 vs. 50 ± 10, respectively; P < 0.001). Wound infection was encountered in two cases in Group I and only one case in Group II (P = 1.000). Follow-up of patients showed an improvement in VAS and ODI scores at 6 months and further decrease at 12 months in the two groups. However, no significant difference was detected between the two groups at 1, 6, or 12 months postoperatively (P > 0.05). No recurrences were detected in patients during the follow-up period of 12 months [
[
[
DISCUSSION
The ILD has been shown to be an effective and safe surgical remedy for herniated lumbar disc.[
The mean operative time in our series was 68 ± 13 min in Group I and was reduced in Group II to 50 ± 4 min. This indicates that the measures adopted in the modified technique helped in reduction of the required time for ILD. These results were supported also by conducting multiple regression analysis that showed a significant association of the modified technique with operative time and intraoperative blood loss, regardless of the level of disc.
The operative time in both groups was shorter than that reported by Wang et al.,[
Xu et al.[
The amount of intraoperative blood loss has not been assessed by most previous studies that evaluated the learning curve of ILD. Intraoperative blood loss was reduced from 50 ± 10 ml in Group I to 42 ± 7 ml in Group II. Xu et al.[
Our results showed that increased experience of the surgical team (with the increased number of operated cases) was significantly and strongly correlated with decrease in the mean total operative time in Group I and the mean amount of intraoperative blood loss in Groups I and II. However, surgical team experience did not correlate significantly with operative time in Group II. It seems that the modified technique enabled the surgical team to attain the required level of proficiency faster, so the plateau of the learning curve was reached early. Consequently, further operated cases did not add significantly to the performance of the team and resulted in non-significant correlation with operative time in Group II.
The severity of pain decreased considerably at 6 and 12 months following surgery. Pre-operative VAS of about 7 ± 1 decreased to 2 ± 1 and 1 ± 1 at 12 months postoperatively in Groups I and II, respectively. Moreover, the mean pre-operative ODI score decreased from 70 ± 6 and 68 ± 8 in Groups I and II, respectively, to reach 16 ± 7 and 14 ± 5 at 12 months postoperatively. These findings indicate that both techniques were comparable in terms of pain control and functional outcome. Hsu et al.[
No specific intraoperative complications were detected in our series, and conversion to open surgery was not mandated in any of our patients. A diversity of reported complications of endoscopic ILD includes unintended durotomy with potential pseudomeningocele, meningitis, and discitis.[
In the present study, the learning curve of endoscopic ILD for operative time in Group I and intraoperative blood loss in Groups I and II was gradual rather than steep, which agrees with the findings of the previous studies reporting endoscopic ILD as a challenging procedure that requires practicing to be mastered. The plateau for the curve of operative time was reached in Group I approximately after the 25th case, which is close to the number of patients (30 patients) recommended for passing the early phase of learning curve in endoscopic procedures.[
A steep learning curve means that the procedure can be mastered after a relatively small number of patients. In this case, the first phase of the curve is short and gives way abruptly to the second phase. In a shallow curve, the first phase is prolonged and gradually merges into the second phase. Therefore, a shallow learning curve indicates that improvement of performance is gradual, and reaching the plateau phase where the procedure is mastered requires practicing on a large number of cases.[
Hsu et al.[
Surgeons performing the procedure during the first phase of the learning curve are susceptible to experience difficulties, resulting in increased risk of complications during this stage and longer operative time than open surgery.[
The interpretation of the learning curve in literature is variable. This could be attributed to the previous experience of surgeons with endoscopic or microscopic procedures and variable levels of herniated disc. Unfortunately, the details of the previous experiences of the surgical teams with endoscopic procedures or open discectomy were not reported by the previous studies to allow comparisons and test this hypothesis. Only Xu et al.[
The present study had points of strength, including a convenient sample size of patients who underwent endoscopic ILD at different disc levels. However, larger sample size is required to describe the learning curve for intraoperative blood loss.
CONCLUSION
In conclusion, the learning curve of endoscopic ILD was shallow with standard technique, indicating difficulties in mastering the procedure and the need of practice on a relatively larger number of patients. Meanwhile, the proposed modified technique helped reaching the required level of proficiency in the early phase of the curve, providing significant reduction in operative time and blood loss, with comparable effectiveness and safety as the standard technique.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al Khathaami AM, Mohammad YO, Alibrahim FS, Jradi HA. Factors associated with late arrival of acute stroke patients to emergency department in Saudi Arabia. SAGE Open Med. 2018. 6: 1-7
2. Al-Eithan MH, Amin M, Robert AA. The effect of hemiplegia/ hemiparesis, diabetes mellitus, and hypertension on hospital length of stay after stroke. Neurosciences (Riyadh). 2011. 16: 253-6
3. Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: A systematic review. Stroke. 2009. 40: 1082-90
4. Benzel EC, Orr RD. A steep learning curve is a good thing!. Spine J. 2011. 11: 131-2
5. Carragee EJ, Han MY, Suen PW, Kim D. Clinical outcomes after lumbar discectomy for sciatica: The effects of fragment type and anular competence. J Bone Joint Surg Am. 2003. 85: 102-8
6. Carragee EJ, Spinnickie AO, Alamin TF, Paragioudakis S. A prospective controlled study of limited versus subtotal posterior discectomy: Short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine (Phila Pa 1976). 2006. 31: 653-7
7. Chae KH, Ju CI, Lee SM, Kim BW, Kim SY, Kim HS. Strategies for noncontained lumbar disc herniation by an endoscopic approach: Transforaminal suprapedicular approach, semi-rigid flexible curved probe, and 3-dimensional reconstruction CT with discogram. J Korean Neurosurg Soc. 2009. 46: 312-6
8. Chang SS, Fu TS, Liang YC, Lia PL, Niu CC, Chen LH. Results of microendoscopic discectomy performed in the 26 cases with a minimum 3 years follow-up. Chang Gung Med J. 2009. 32: 89-97
9. Choi G, Lee SH, Raiturker PP, Lee S, Chae YS. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery. 2006. 58: ONS59-68
10. Choi G, Prada N, Modi HN, Vasavada NB, Kim JS, Lee SH. Percutaneous endoscopic lumbar herniectomy for high-grade down-migrated L4-L5 disc through an L5-S1 interlaminar approach: A technical note. Minim Invasive Neurosurg. 2010. 53: 147-52
11. Chumnanvej S, Kesornsak W, Sarnvivad P, Paiboonsirijit S, Kuansongthum V. Full endoscopic lumbar discectomy via interlaminar approach: 2-year results in Ramathibodi Hospital. J Med Assoc Thai. 2011. 94: 1465-70
12. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980. 66: 271-3
13. Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: Updated cochrane review. Spine (Phila Pa 1976). 2007. 32: 1735-47
14. Gotfryd A, Avanzi O. A systematic review of randomised clinical trials using posterior discectomy to treat lumbar disc herniations. Int Orthop. 2009. 33: 11-7
15. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken). 2011. 63: S240-52
16. Hsu HT, Chang SJ, Yang SS, Chai CL. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J. 2013. 22: 727-33
17. Inui A, Sairyo K, Katoh S, Higashino K, Sakai T, Shiiba M. Extruded lumbar osseous endplate causing long-term radiculopathy in an adult: An endoscopic excision. Minim Invasive Neurosurg. 2006. 49: 55-7
18. Jhala A, Mistry M. Endoscopic lumbar discectomy: Experience of first 100 cases. Indian J Orthop. 2010. 44: 184-90
19. Kim CH, Chung CK, Jahng TA, Yang HJ, Son YJ. Surgical outcome of percutaneous endoscopic interlaminar lumbar diskectomy for recurrent disk herniation after open diskectomy. J Spinal Disord Tech. 2012. 25: E125-33
20. Kim HS, Ju CI, Kim SW, Kim JG. Endoscopic transforaminal suprapedicular approach in high grade inferior migrated lumbar disc herniation. J Korean Neurosurg Soc. 2009. 45: 67-73
21. Lee DY, Ahn Y, Lee SH. Percutaneous endoscopic lumbar discectomy for adolescent lumbar disc herniation: Surgical outcomes in 46 consecutive patients. Mt Sinai J Med. 2006. 73: 864-70
22. Matsumoto M, Watanabe K, Tuji T, Ishii K, Takaishi H, Nakamura M. Microendoscopic discectomy for lumbar disc herniation with bony fragment due to apophyseal separation. Minim Invasive Neurosurg. 2007. 50: 335-9
23. Righesso O, Falavigna A, Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: Results of a randomized controlled trial. Neurosurgery. 2007. 61: 545-9
24. Ruetten S, Komp M, Godolias G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: Prospective 2-year results of 331 patients. Minim Invasive Neurosurg. 2006. 49: 80-7
25. Ruetten S, Komp M, Godolias G. An extreme lateral access for the surgery of lumbar disc herniations inside the spinal canal using the full-endoscopic uniportal transforaminal approach-technique and prospective results of 463 patients. Spine (Phila Pa 1976). 2005. 30: 2570-8
26. Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: A prospective, randomized, controlled study. Spine (Phila Pa 1976). 2008. 33: 931-9
27. Ruetten S, Komp M, Merk H, Godolias G. Recurrent lumbar disc herniation after conventional discectomy: A prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech. 2009. 22: 122-9
28. Ruetten S, Komp M, Merk H, Godolias G. Use of newly developed instruments and endoscopes: Full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine. 2007. 6: 521-30
29. Ruetten S. Full-endoscopic operations of the spine in disk herniations and spinal stenosis. Surg Technol Int. 2011. 21: 284-98
30. Sencer A, Yorukoglu AG, Akcakaya MO, Aras Y, Aydoseli A, Boyali O. Fully endoscopic interlaminar and transforaminal lumbar discectomy: Short-term clinical results of 163 surgically treated patients. World Neurosurg. 2014. 82: 884-90
31. Shin KH, Chang HG, Rhee NK, Lim KS. Revisional percutaneous full endoscopic disc surgery for recurrent herniation of previous open lumbar discectomy. Asian Spine J. 2011. 5: 1-9
32. Smith JS, Ogden AT, Shafizadeh S, Fessler RG. Clinical outcomes after microendoscopic discectomy for recurrent lumbar disc herniation. J Spinal Disord Tech. 2010. 23: 30-4
33. Teli M, Lovi A, Brayda-Bruno M, Zagra A, Corriero A, Giudici F. Higher risk of dural tears and recurrent herniation with lumbar micro-endoscopic discectomy. Eur Spine J. 2010. 19: 443-50
34. Tonogai I, Sairyo K, Higashino K, Sakai T, Katoh S, Yasui N. Minimally invasive endoscopic removal of a herniated nucleus pulposus that had migrated to the S1 nerve root foramen. Minim Invasive Neurosurg. 2007. 50: 173-7
35. Wang B, Lu G, Patel AA, Ren P, Cheng I. An evaluation of the learning curve for a complex surgical technique: The full endoscopic interlaminar approach for lumbar disc herniations. Spine J. 2011. 11: 122-30
36. Xu H, Liu X, Liu G, Zhao J, Fu Q, Xu B. Learning curve of full-endoscopic technique through interlaminar approach for L5/ S1 disk herniations. Cell Biochem Biophys. 2014. 70: 1069-74
37. Yadav YR, Parihar V, Kher Y, Bhatele PR. Endoscopic inter laminar management of lumbar disease. Asian J Neurosurg. 2016. 11: 1-7
38. Yaqub BA, Shamena AR, Kolawole TM, Patel PJ. Cerebrovascular disease in Saudi Arabia. Stroke. 1991. 22: 1173-6
39. Yoshimoto M, Takebayashi T, Kawaguchi S, Tsuda H, Ida K, Wada T. Minimally invasive technique for decompression of lumbar foraminal stenosis using a spinal microendoscope: Technical note. Minim Invasive Neurosurg. 2011. 54: 142-6
40. Zhou Y, Wang M, Wang J, Chu TW, Zhang ZF, Li CQ. Clinical experience and results of lumbar microendoscopic discectomy: A study with a five-year follow-up. Orthop Surg. 2009. 1: 171-5