- Department of Neurosurgery, Nagoya University Graduate School of Medicine, Nagoya, Japan,
- Department of Skull Base Surgery, Republican Specialized Scientific Practical Medical Center of Neurosurgery, Tashkent, Uzbekistan,
- Department of Neurosurgery, Aichi Medical University, Nagoya, Japan,
- Department of Neurosurgery, Tashkent Medical Academy, Tashkent, Uzbekistan.
Correspondence Address:
Maruf Matmusaev, Department of Skull Base Surgery, Republican Specialized Scientific Practical Medical Center of Neurosurgery, Tashkent, Uzbekistan.
DOI:10.25259/SNI_1102_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Maruf Matmusaev1,2, Tadashi Watanabe3, Kenichiro Iwami3, Tokhir Akhmediev4. Endoscopic transnasal transsphenoidal management of sellar/suprasellar arachnoid cyst: A case report and literature review. 07-Apr-2023;14:131
How to cite this URL: Maruf Matmusaev1,2, Tadashi Watanabe3, Kenichiro Iwami3, Tokhir Akhmediev4. Endoscopic transnasal transsphenoidal management of sellar/suprasellar arachnoid cyst: A case report and literature review. 07-Apr-2023;14:131. Available from: https://surgicalneurologyint.com/surgicalint-articles/endoscopic-transnasal-transsphenoidal-management-of-sellar-suprasellar-arachnoid-cyst-a-case-report-and-literature-review/
Abstract
Background: Arachnoid cysts (ACs) are collections of cerebrospinal fluids (CSFs) that develop within the arachnoid layer of the meninges. Sellar ACs are comparatively rare. In general, ACs account for approximately 1% of all intracranial mass lesions, and sellar ACs are 3% of all intracranial ACs. An endoscopic transnasal transsphenoidal approach for the treatment of ACs by fenestrating the cyst’s wall and connecting with the subarachnoid space is the most optimal option.
Case Description: A 74-year-old woman whose sellar AC was diagnosed on magnetic resonance imaging a year ago was admitted to our hospital with complaints of bitemporal hemianopia and diminished visual acuity in the past 2 months. Sellar AC was diagnosed based on the clinical history and presentation, as well as neurologic, endocrinologic, and ophthalmologic examinations, including visual acuity and visual field examination, and additional imaging findings. The patient with a sellar/suprasellar AC was treated by an endoscopic transnasal transsphenoidal approach with cyst drainage and perforation of the lamina terminalis. Postoperatively, the visual disturbances improved markedly. No surgery-related complications occurred.
Conclusion: The endoscopic transnasal transsphenoidal approach remains a minimally invasive and preferred approach for the treatment of sellar/suprasellar ACs. Hermetically reconstructing the sellar floor is an effective method to prevent CSF leakage.
Keywords: Cerebrospinal fluid leak, Endonasal transsphenoidal surgery, Sellar/suprasellar arachnoid cyst
INTRODUCTION
Arachnoid cysts (ACs) are collections of cerebrospinal fluids (CSFs) that develop within the arachnoid layer of the meninges. The cysts can develop at any place in the subarachnoid space along the cerebrospinal axis.[
The purpose of the surgery for sellar ACs is to relieve symptoms and prevent complications. An endoscopic transnasal transsphenoidal approach for the treatment of ACs by fenestrating the cyst’s wall and connecting with the subarachnoid space is the most optimal option.
A postoperative CSF leakage is a major complication of endoscopic transsphenoidal surgery (TS).[
Many endoscopic neurosurgeons have reported different reconstructions of the skull base to prevent CSF leakage after endoscopic TS. Using fat graft, fascia lata, sphenoidal mucosa,[
Dural suturing is considered the safest method for the reconstruction of the skull base according to reports from some authors.[
Endoscope use in minimally invasive neurosurgery of the sellar and parasellar lesions has become an effective tool due to the panoramic views, bright lighting, and no blocks or dead corner in the surgical field. Moreover, an angled endoscope has the advantage of inspection beyond anatomic barriers and regions that will not allow a conventional operative microscope.[
In this paper, we describe a case of symptomatic sellar AC treated by endoscopic TS using fenestration in the subarachnoid space to prevent CSF leakage by continuous dural suturing with 6-0 monofilament sutures using an abdominal fat graft.
CASE DESCRIPTION
A 74-year-old woman whose sellar AC was diagnosed on magnetic resonance imaging (MRI) a year ago was admitted to our hospital with complaints of bitemporal hemianopia and diminished visual acuity in the past 2 months. Her medical history revealed that she had chronic arterial hypertension. Sellar AC was diagnosed based on the clinical history and presentation and neurologic, endocrinologic, and ophthalmologic examinations, including visual acuity and visual field examination, and additional imaging findings.
The pituitary function was assessed using standard hormonal tests, such as the levels of thyroid-stimulating hormones and thyroxine (free-T3 and free-T4), growth hormones and insulin-like growth factor-1, plasma adrenocorticotropic hormones and serum cortisol, prolactin, luteinizing hormones, and follicular-stimulating hormones. The patient’s basal serum anterior pituitary hormone levels were within normal limits.
Visual function assessment involved measuring visual acuity using standard visual field testing. Visual field tests revealed bitemporal hemianopia and diminished visual acuity.
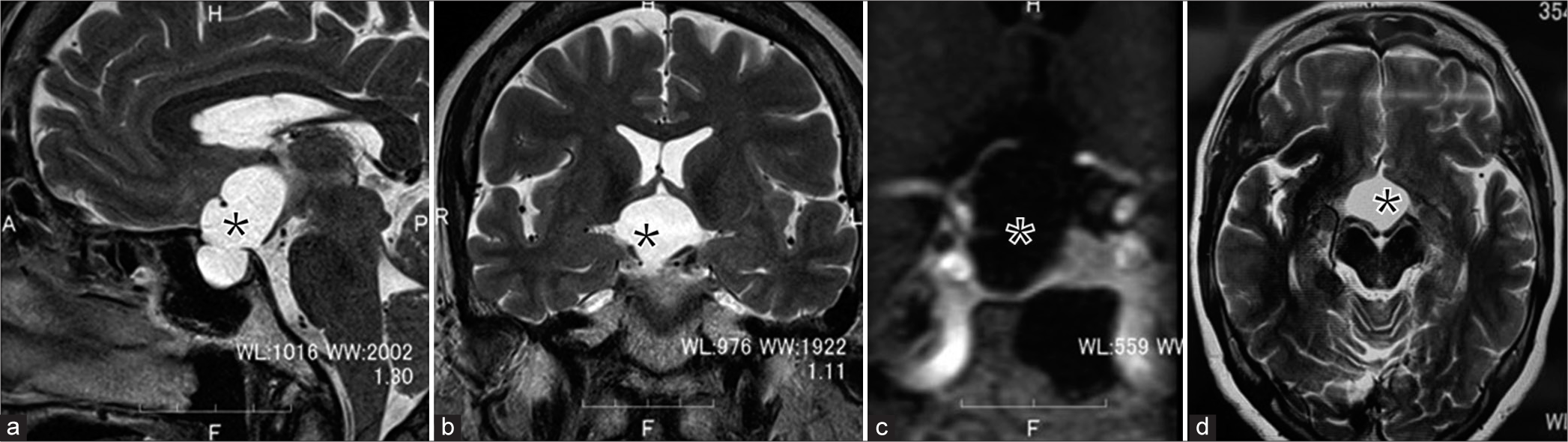
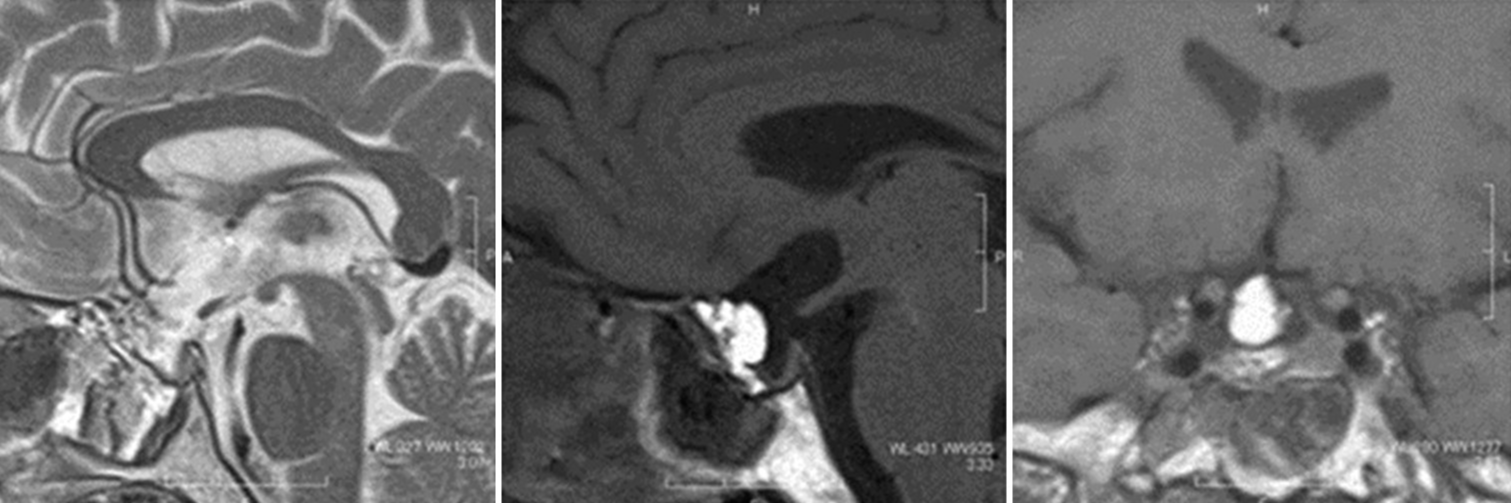
The radiologic diagnosis of a sellar AC is provided essentially by an MRI scan. MRI identified a well-defined, homogeneous, cystic sellar lesion with suprasellar extension and thin walls. The pituitary gland and stalk appeared stretched over the borders of the cyst and compressed against the dorsum sellae. No calcifications or solid areas were identified. On the MRI, a cystic lesion with dimensions of 30 × 15 × 17 mm and cystic mass content appeared isointense to the CSF. The optic chiasm (OCh) was compressed [
Figure 1:
Preoperative pituitary magnetic resonance imaging showing an intrasuprasellar Arachnoid cyst. (a-c) Sagittal and coronal views reveal a large hyperintense and well-delineated sellar lesion (asterisk) with significant suprasellar extension. (d) Axial view. *This lesion occupies the whole sella turcica.
Surgical procedures
An endoscopic endonasal approach is considered the first-line treatment for this lesion. The necessary tools for performing surgery are: 4 mm in diameter, 18 cm in length, with 0°, 30°, and 70° rigid endoscopes (Karl Storz, Tuttlingen, Germany), which are held by a floor-standing pneumatic holder (Uniarm [Mitaka Kohki, Tokyo, Japan]). They are designed to move smoothly and steadily.
Under general anesthesia the patient was placed supine position with head raised 20° and fixed on a circle pillow. Extended endoscopic endonasal transsphenoidal approach with the navigational guidance was performed to the cystic sellar lesion.
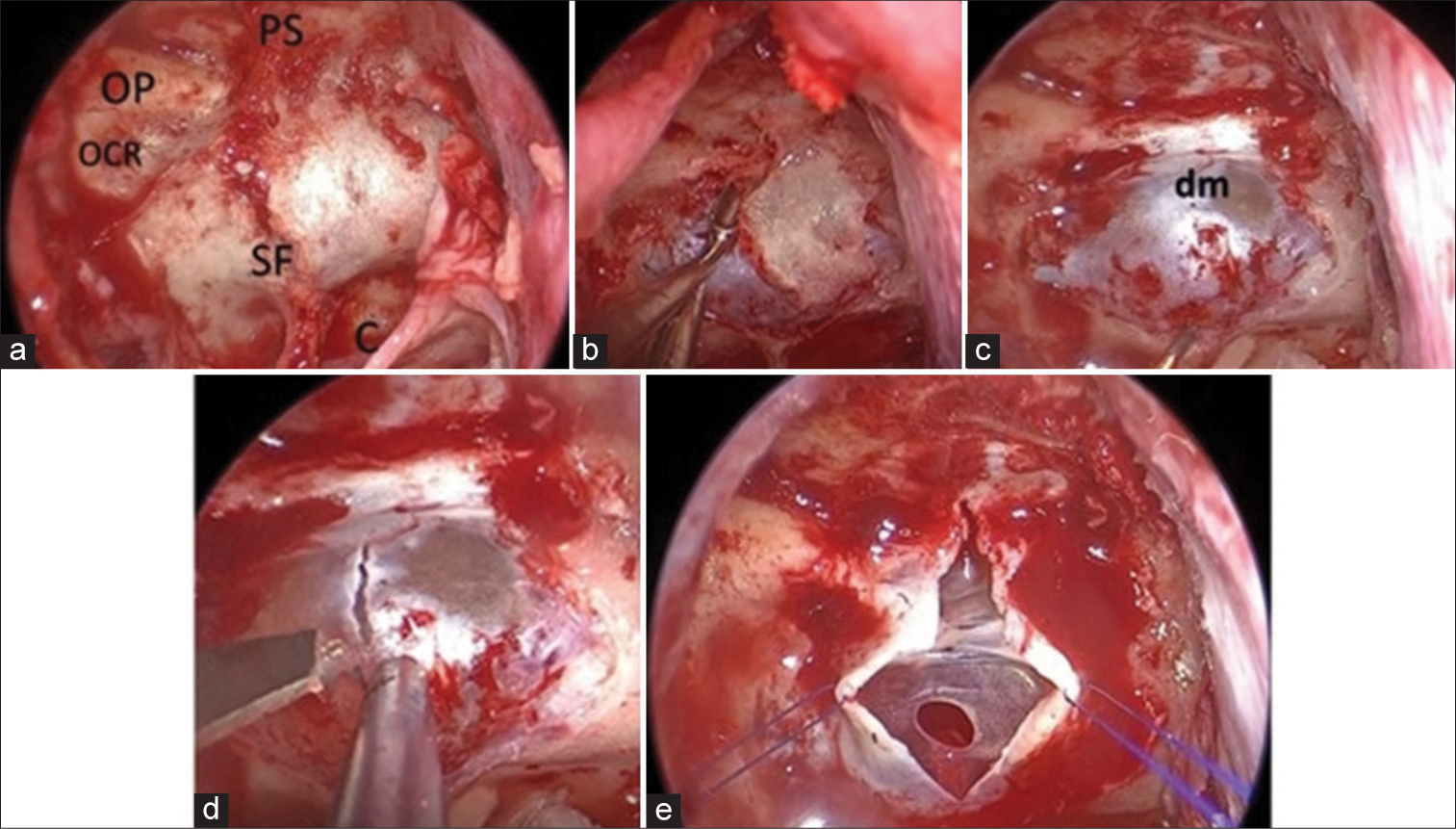
After applying the nasal decongestant, the Ear, nose, and throat (ENT) surgeon started the operation from the left nostril. A submucosal-transseptal approach was performed. A mucosal incision was made near the mucosal junction, the nasal septum resected, the anterior wall of the sphenoid sinus opened, the septum removed, and the left sphenoid sinus mucosa turned downward. After accessing the sella, the neurosurgeon started the next step of the procedure with the assistance of the ENT surgeon and guiding endoscope. The septum was drilled, and the sella turcica, sellar tuberculum, and planum sphenoidale were drilled out after confirming the navigation. The upper part of the optic canal was carefully drilled out with a 3 mm drill. After a wide sphenoidotomy and sellar bone opening, a vertical dural incision was made, dural leaves lateralized to the sides using two Prolene 6.0 sutures, and then the AC was confirmed [
Figure 2:
Sellar stage. (a and b) Opening of the sellar floor, tuberculum sella, and planum sphenoidale were removed. (c) After opening the sellar floor, the dura mater was exposed. (d and e) Vertical incision of the dura mater using a surgical knife. Dural leaves were lateralised to the sides using two Prolene 6.0 sutures, and an Arachnoid cyst was confirmed. OP: Optic prominence, OCR: Optocarotid recess, SF: Sellar floor, PS: Planum sphenoidale, C: Clivus, dm: Dura mater.
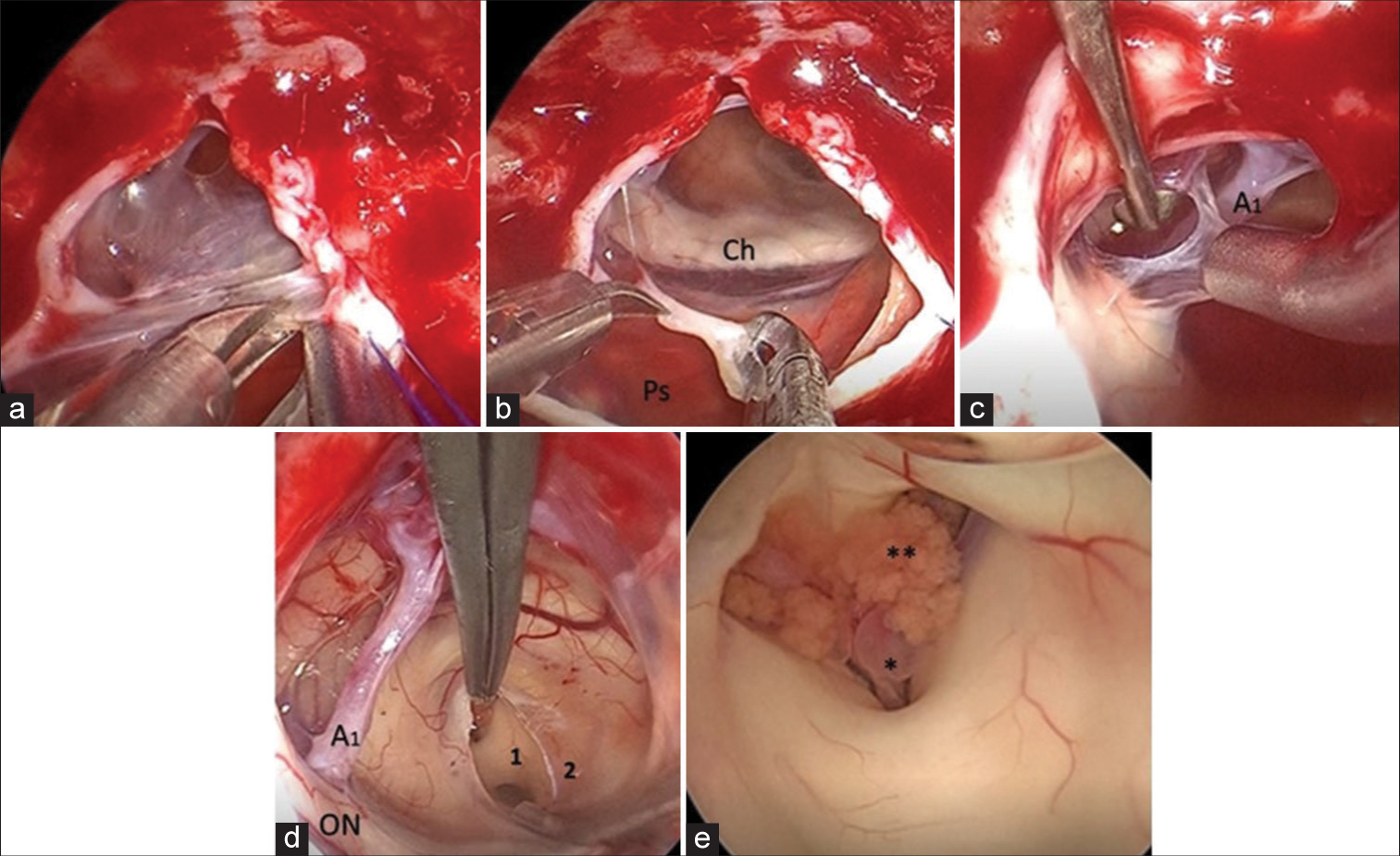
When the walls of the AC were opened, the pituitary gland, pituitary stalk, and OCh were visualized. The anterior communicating artery (Acom), right anterior cerebral artery (A1), and OCh were also clearly observed [
Figure 3:
Intraoperative images.(a) Excision of the left anterior part of the cyst wall. (b) Tissue collection. The pituitary stalk, optic chiasm, and right anterior cerebral artery (A1) were identified. (c) The anterior cyst wall was gradually dissected and removed from the right A1. (d) The lamina terminalis was coagulated by bipolar and fenestrated using cautery and scissors. (e) Foramen of Monroe (Wet field). Ch: Optic chiasm, Ps: Pituitary stalk, ON: Optic nerve. 1: Massa intermedia, 2: Lamina terminalis, *Thalamic veins, **Choroid plexus.
Next, the thinned lamina terminalis was identified. This membrane was coagulated by bipolar and fenestrated using cautery and scissors. Then, the cavity of the third ventricle was inspected, and the massa intermedia, foramen of Monro, and choroid plexus, as well as thalamic veins, were visualized [
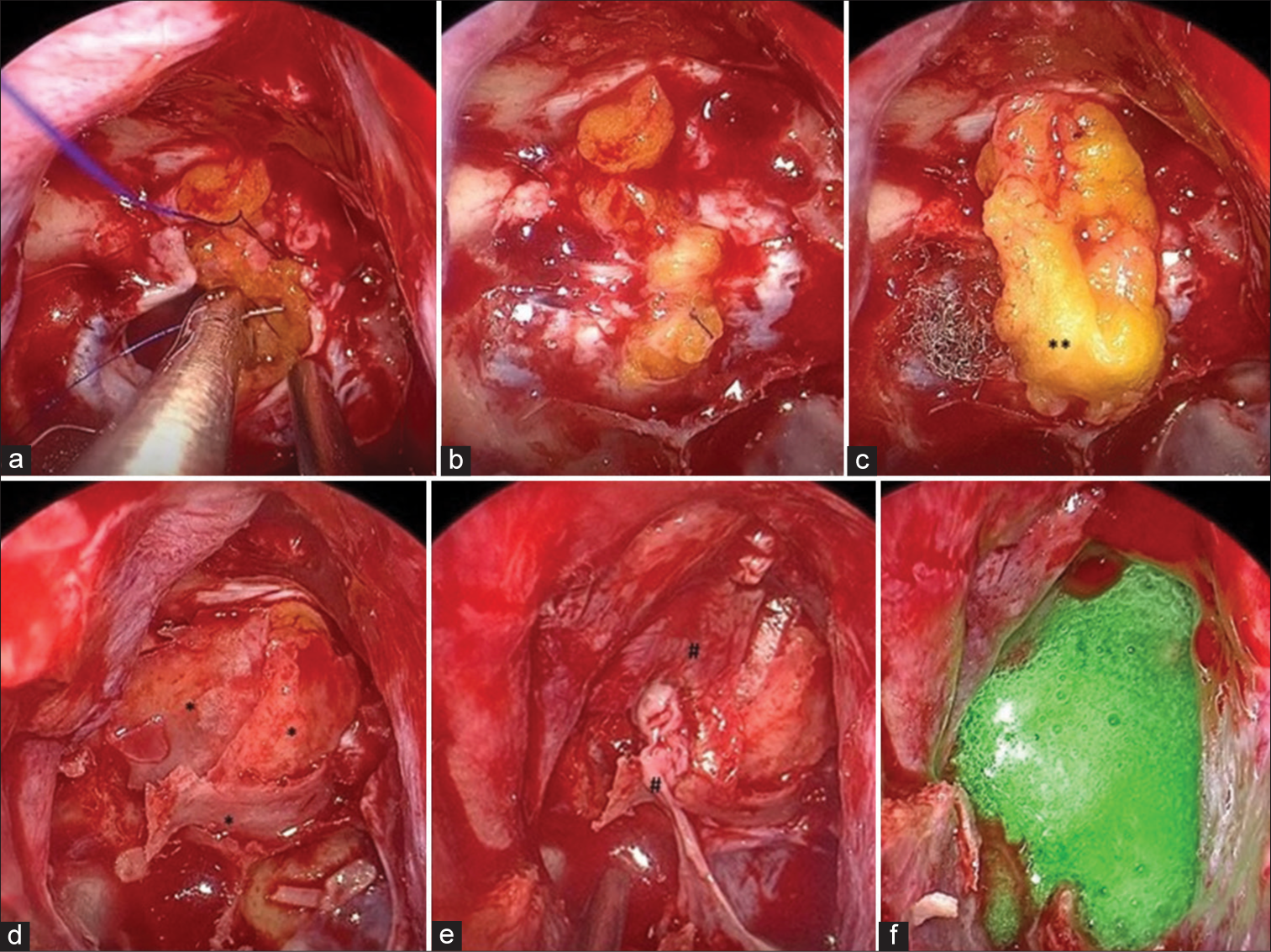
After confirming a good passage of CSF through the fenestration, the dura was closed. For closure, abdominal fat was taken and sutured continuously with 6-0 Prolene to the edges of the dura. We used another piece of compressed fat above the incision line as an onlay-graft. Then, the bony septum was divided into three pieces and laid on the fat graft and the sphenoid sinus mucosa and the upper right septal mucosa were overlaid to reconstruct the bony defect finally fixed with a dural sealant [
Figure 4:
Reconstruction technique. Multilayer technique. Intraoperative views. (a) The view during the dural suturing. Continuous dural suture with abdominal fat, (b) The view after dural suturing, (c) Onlay fat. The dural opening is completely covered using fat, (d) Bone fragments are applied to the fat tissue, (e) The mucous membrane of the sphenoid sinus and right septum is used for the reconstruction of the sellae, (f) Synthetic dural sealant. *Bony septum fragments, **Abdominal fat,
The patient underwent endoscopic endonasal surgery, and the lesion was decompressed and fenestrated. Postoperative patient’s condition was without complications. Her visual disturbances were markedly improved and a pathological examination of the walls confirmed the diagnosis of an AC. No new endocrine abnormalities, CSF leak, meningitis, or neurological symptoms occurred. Follow-up (FU) MRI revealed a reduced size of the mass [
Figure 5:
Postoperative magnetic resonance imaging shows that the sellar arachnoid cyst has markedly decreased in size. The intrasellar part of the cyst covered by normal pituitary tissue is evident, and chiasma is not compressed. Fat graft is visualized as hyperintensity on T1 sagittal and coronal images.
DISCUSSION
In normal conditions, there is no arachnoid tissue below the diaphragma sellae, and the pathophysiology of the development of sellar/suprasellar ACs remains controversial. Several theories may explain and suggest the formation of sellar/suprasellar ACs. It has been suggested that sellar/suprasellar ACs arise from a defect in the diaphragm sellae, through which the basal arachnoid membrane prolapses. Consequently, sellar ACs begin as an intrasellar arachnoid diverticulum, forming a cyst by a narrowing of the diaphragm defect or adhesion formation between the arachnoid layers.[
Radiologically, sellar/suprasellar ACs are diagnosed mainly by MRI scans. The main characteristics of MRI scans of sellar/ suprasellar ACs are cystic lesions with suprasellar extension, balloon-shape, good-delineation, homogeneity, no contrast enhancement and calcification, molding without invading the cavernous sinus, intensity similar to CSF, hypointense T1-weighted signal, and a hyperintense T2-weighted signal on the MRI images.[
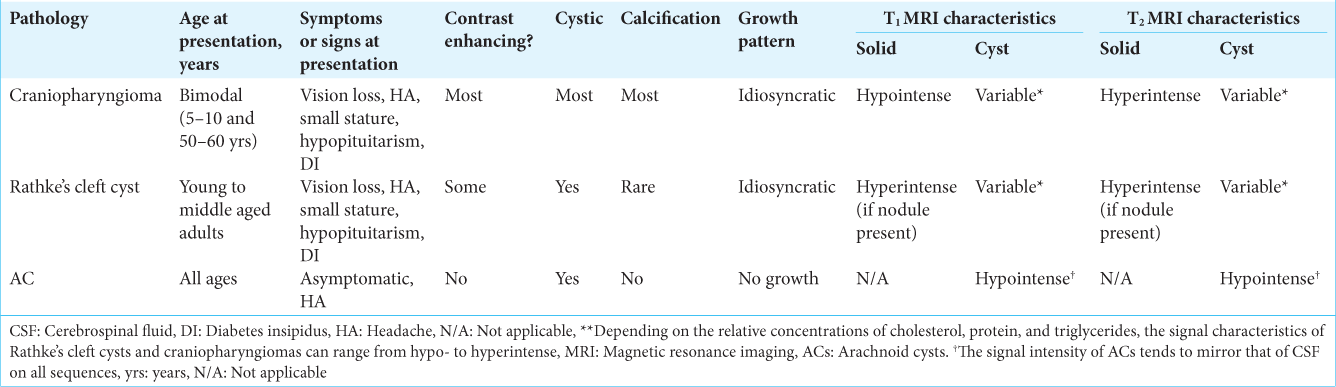
In the radiological differential diagnosis of sellar/suprasellar cystic lesions, Rathke’s cleft cysts or craniopharyngiomas should be considered, being significant for preoperative planning. The differential diagnosis for sellar/suprasellar ACs in adults is summarized in
The defining characteristics of craniopharyngiomas on the MRI are heterogeneous, mixed (mostly solid and cystic), calcified, and contrast-enhanced imaging features. The solid portion contains a hypointense T1-weighted signal, while the cystic portion’s signal is changeable.[
The indications for surgery for sellar/suprasellar ACs include pituitary dysfunction, visual disturbance, and severe headaches. The surgery for the treatment of sellar ACs aims at the fenestration of the cyst wall to communicate between the cyst and subarachnoid space, as well as the decompression of the visual apparatus and the pituitary gland. Adequate therapy is the excision of the cyst wall, which will allow histological analysis to identify the lesion’s pathology. Total removal of the walls of the cyst, to prevent damage to the pituitary gland is unnecessary.
Several surgical approaches have been described for treating sellar ACs, such as transcranial (pterional, frontotemporal, orbitofrontal, or subfrontal) and transsphenoidal (endoscopic or microscopic) approaches.[
Percutaneous ventriculocystostomy,[
In the treatment of sellar ACs, the endoscopic transsphenoidal approach is less invasive than transcranial approaches. Moreover, the endoscope provides better visualization of the supra- and parasellar spaces.[
Due to its advantages, the transsphenoidal transnasal approach using a microscope or endoscope is the most reasonable option for the treatment of sellar ACs. Below is described the work of several authors who have applied the method of TS in the treatment of these cysts.[
In most clinical cases, until recently, the microscopic transsphenoidal approach was used more often, but Cavallo et al. mentioned the advantage of the endoscope in the transsphenoidal treatment of sellar cystic lesions. Due to its panoramic view, the endoscope allows a much wider visualization of the suprasellar cistern and the entire cavity, which enables better examination of the cyst cavity, by studying all the walls of the cyst, to exclude signs of a cystic tumor.[
In the treatment of intrasellar arachnoid cyst by TS, the intrasellar part of the cyst is drained, to prevent CSF leakage and recurrence, sellar floor is filled with fat or muscle.
A preoperative sagittal view of an MRI of the brain must be carefully examined to determine the position of the pituitary stalk and the relationship between the vascular structures behind the dorsum sellae.[
Oyama et al. described the method based on an extended TS for supra- and parasellar lesions using a microscope. This work described a keyhole dural incision wide enough to safely perform a cyst cisternostomy using an angled endoscope. The dural defect was reconstructed using fascia lata and six to eight sutures with 6-0 nylon monofilament. Moreover, the use of the 30° or 70° angled thin endoscope enables the determination of the position of the Acom complex and the optic apparatus, to avoid iatrogenic damage. One patient had CSF leakage and meningitis, which were successfully treated with conservative therapy with lumbar drainage (LD) and intravenous antibiotics. Out of the seven cases, there was no recurrence, and no reoperation was required for CSF leakage. Preoperative visual disturbance improved after surgery.[
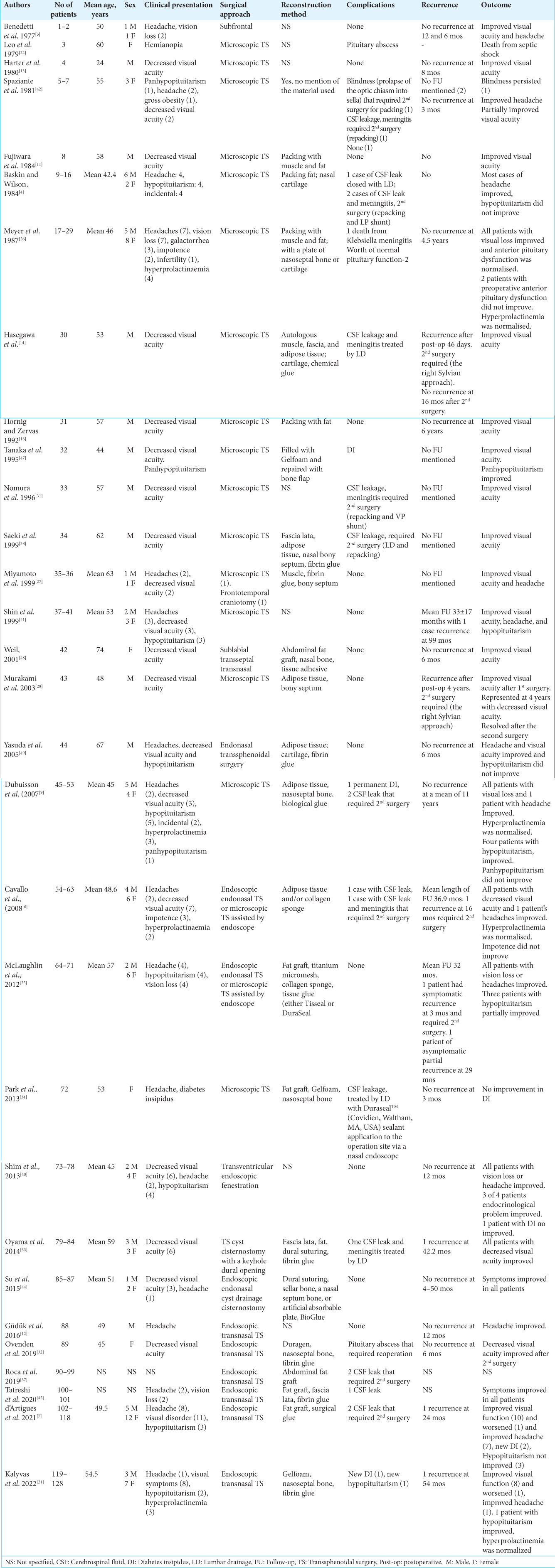
The clinical presentation of 128 cases with sellar/suprasellar ACs is summarized in
Postoperative CSF leakage is a major complication of TS. Eighteen of the 128 reported cases developed CSF leakage, with most of them requiring second surgery or LD.[
In most cases, the surgical treatment of sellar/suprasellar ACs is successful, but according to some sources, there are reports of recurrence.[
CONCLUSION
The endoscopic transnasal transsphenoidal approach remains a minimally invasive and the preferred approach for the treatment of sellar/suprasellar ACs. To maintain the patency of CSF flow through the AC, the lamina terminalis was perforated in addition to the anterior wall fenestration. This perforation can prevent the recurrence of the AC. Hermetically reconstructing the sellar floor and continuous dural suturing with fat graft is effective in preventing CSF leakage and does not require additional procedures such as the placement of a lumbar drain and the insertion of a nasal septal flap.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflict of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aljuboori Z, Burke W, Yeo H, McCallum A, Clark J, Williams B. Orbitofrontal approach for the fenestration of a symptomatic sellar arachnoid cyst. Surg Neurol Int. 2020. 11: 10
2. Amano K, Hori T, Kawamata T, Okada Y. Repair and prevention of cerebrospinal fluid leakage in transsphenoidal surgery: A sphenoid sinus mucosa technique. Neurosurg Rev. 2016. 39: 123-31 discussion 131
3. Aoki N, Sakai T. Intraoperative subdural hematoma in a patient with arachnoid cyst in the middle cranial fossa. Childs Nerv Syst. 1990. 6: 44-6
4. Baskin DS, Wilson CB. Transsphenoidal treatment of nonneoplastic intrasellar cysts. A report of 38 cases. J Neurosurg. 1984. 60: 8-13
5. Benedetti A, Carbonin C, Colombo F. Possible aetiopathogenetic correlation between primary empty sella and arachnoid cyst. Acta Neurochir (Wien). 1977. 38: 269-78
6. Cavallo LM, Prevedello D, Esposito F, Laws ER, Dusick JR, Messina A. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008. 31: 55-64 discussion 64
7. d’Artigues J, Graillon T, Boissonneau S, Farah K, Amodru V, Brue T. Fully endoscopic endonasal approach for the treatment of intrasellar arachnoid cysts. Pituitary. 2022. 25: 191-200
8. Dawkins RL, Hackney JR, Riley KO. Penetration of an optic nerve by a sellar/suprasellar arachnoid cyst. World Neurosurg. 2016. 87: 662.e7-11
9. Dubuisson AS, Stevenaert A, Martin DH, Flandroy PP. Intrasellar arachnoid cysts. Neurosurgery. 2007. 61: 505-13
10. Elliott RE, Tanweer O, Rubin BA, Koslow M, Mikolaenko I, Wisoff JH. Suprasellar hamartoma and arachnoid cyst. World Neurosurg. 2013. 80: e401-7
11. Fujiwara M, Bitoh S, Hasegawa H, Ohtsuki H. A case of intrasellar arachnoid cyst. No Shinkei Geka. 1984. 12: 331-7
12. Güdük M, HamitAytar M, Sav A, Berkman Z. Intrasellar arachnoid cyst: A case report and review of the literature. Int J Surg Case Rep. 2016. 23: 105-8
13. Harter LP, Silverberg GD, Brant-Zawadzki M. Intrasellar arachnoid cyst: Case report. Neurosurgery. 1980. 7: 387-90
14. Hasegawa M, Yamashima T, Yamashita J, Kuroda E. Symptomatic intrasellar arachnoid cyst: Case report. Surg Neurol. 1991. 35: 355-9
15. Horiguchi K, Murai H, Hasegawa Y, Hanazawa T, Yamakami I, Saeki N. Endoscopic endonasal skull base reconstruction using a nasal septal flap: Surgical results and comparison with previous reconstructions. Neurosurg Rev. 2010. 33: 235-41
16. Hornig GW, Zervas NT. Slit defect of the diaphragma sellae with valve effect: Observation of a “slit valve”. Neurosurgery. 1992. 30: 265-7
17. Iqbal J, Kanaan I, Al Homsi M. Non-neoplastic cystic lesions of the sellar region presentation, diagnosis and management of eight cases and review of the literature. Acta Neurochir (Wien). 1999. 141: 389-97
18. Ishii Y, Tahara S, Oyama K, Kitamura T, Teramoto A. Easy slip-knot: A new simple tying technique for deep sutures. Acta Neurochir (Wien). 2011. 153: 1543-5 discussion 1545
19. Ishikawa T, Takeuchi K, Nagata Y, Choo J, Kawabata T, Ishizaki T. Three types of dural suturing for closure of CSF leak after endoscopic transsphenoidal surgery. J Neurosurg. 2019. 131: 1625-31
20. Jho HD, Carrau RL. Endoscopy assisted transsphenoidal surgery for pituitary adenoma. Technical note. Acta Neurochir (Wien). 1996. 138: 1416-25
21. Kalyvas A, Milesi M, Leite M, Yang K, St Jacques L, Vescan A. Endoscopic treatment of sellar arachnoid cysts via a simple cyst-opening technique: Long-term outcomes from a single center. World Neurosurg. 2022. 161: e625-34
22. Leo JS, Pinto RS, Hulvat GF, Epstein F, Kricheff II. Computed tomography of arachnoid cysts. Radiology. 1979. 130: 675-80
23. Mangussi-Gomes J, Gentil AF, Filippi RZ, Momesso RA, Handfas BW, Radvany J. Sellar and suprasellar arachnoid cyst. Einstein (Sao Paulo). 2019. 17: eAI4269
24. Matmusaev M, Kumar RS, Yamada Y, Nagatani T, Kawase T, Tanaka R. Endoscopic microvascular decompression for hemifacial spasm. Asian J Neurosurg. 2020. 15: 833-8
25. McLaughlin N, Vandergrift A, Ditzel Filho LF, Shahlaie K, Eisenberg AA, Carrau RL. Endonasal management of sellar arachnoid cysts: Simple cyst obliteration technique. J Neurosurg. 2012. 116: 728-40
26. Meyer FB, Carpenter SM, Laws ER. Intrasellar arachnoid cysts. Surg Neurol. 1987. 28: 105-10
27. Miyamoto T, Ebisudani D, Kitamura K, Ohshima T, Horiguchi H, Nagahiro S. Surgical management of symptomatic intrasellar arachnoid cysts-two case reports. Neurol Med Chir (Tokyo). 1999. 39: 941-5
28. Murakami M, Okumura H, Kakita K. Recurrent intrasellar arachnoid cyst. Neurol Med Chir (Tokyo). 2003. 43: 312-5
29. Nagata Y, Watanabe T, Nagatani T, Takeuchi K, Chu J, Wakabayashi T. The multiscope technique for microvascular decompression. World Neurosurg. 2017. 103: 310-4
30. Nishioka H, Izawa H, Ikeda Y, Namatame H, Fukami S, Haraoka J. Dural suturing for repair of cerebrospinal fluid leak in transnasal transsphenoidal surgery. Acta Neurochir (Wien). 2009. 151: 1427-30
31. Nomura M, Tachibana O, Hasegawa M, Kohda Y, Nakada M, Yamashima T. Contrast-enhanced MRI of intrasellar arachnoid cysts: Relationship between the pituitary gland and cyst. Neuroradiology. 1996. 38: 566-8
32. Ovenden CD, Almeida JP, Oswari S, Gentili F. Pituitary abscess following endoscopic endonasal drainage of a suprasellar arachnoid cyst: Case report and review of the literature. J Clin Neurosci. 2019. 68: 322-8
33. Oyama K, Fukuhara N, Taguchi M, Takeshita A, Takeuchi Y, Yamada S. Transsphenoidal cyst cisternostomy with a keyhole dural opening for sellar arachnoid cysts: Technical note. Neurosurg Rev. 2014. 37: 261-7
34. Park KH, Gwak HS, Hong EK, Lee SH. Inflamed symptomatic sellar arachnoid cyst: Case report. Brain Tumor Res Treat. 2013. 1: 28-31
35. Pierre-Kahn A, Capelle L, Brauner R, Sainte-Rose C, Renier D, Rappaport R. Presentation and management of suprasellar arachnoid cysts. Review of 20 cases. J Neurosurg. 1990. 73: 355-9
36. Rappaport ZH. Suprasellar arachnoid cysts: Options in operative management. Acta Neurochir (Wien). 1993. 122: 71-5
37. Roca E, Penn DL, Safain MG, Burke WT, Castlen JP, Laws ER. Abdominal fat graft for sellar reconstruction: Retrospective outcomes review and technical note. Oper Neurosurg (Hagerstown). 2019. 16: 667-74
38. Saeki N, Tokunaga H, Hoshi S, Sunada S, Sunami K, Uchino F. Delayed postoperative CSF rhinorrhea of intrasellar arachnoid cyst. Acta Neurochir (Wien). 1999. 141: 165-9
39. Shim KW, Lee YH, Park EK, Park YS, Choi JU, Kim DS. Treatment option for arachnoid cysts. Childs Nerv Syst. 2009. 25: 1459-66
40. Shim KW, Park EK, Lee YH, Kim SH, Kim DS. Transventricular endoscopic fenestration of intrasellar arachnoid cyst. Neurosurgery. 2013. 72: 520-8
41. Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S. Cystic lesions of the pituitary: Clinicopathological features distinguishing craniopharyngioma, Rathke’s cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab. 1999. 84: 3972-82
42. Spaziante R, de Divitiis E, Stella L, Cappabianca P, Donzelli R. Benign intrasellar cysts. Surg Neurol. 1981. 15: 274-82
43. Stein SC. Intracranial developmental cysts in children: Treatment by cystoperitoneal shunting. Neurosurgery. 1981. 8: 647-50
44. Su Y, Ishii Y, Lin CM, Tahara S, Teramoto A, Morita A. Endoscopic transsphenoidal cisternostomy for nonneoplastic sellar cysts. Biomed Res Int. 2015. 2015: 389474
45. Tafreshi AR, Du R, Rutkowski MJ, Donoho DA, Shiroishi MS, Liu CJ. Differential clinical presentation, intraoperative management strategies, and surgical outcomes after endoscopic endonasal treatment of cystic sellar masses. World Neurosurg. 2020. 133: e241-51
46. Takeuchi K, Nagatani T, Wakabayashi T. How I do it: Shoelace watertight dural closure in extended transsphenoidal surgery. Acta Neurochir (Wien). 2015. 157: 2089-92
47. Tanaka Y, Hayashi S, Nakai M, Ryujin Y, Uematsu Y, Nakai K. Intrasellar arachnoid cyst: A case report. No Shinkei Geka. 1995. 23: 801-6
48. Weil RJ. Rapidly progressive visual loss caused by a sellar arachnoid cyst: Reversal with transsphenoidal microsurgery. South Med J. 2001. 94: 1118-21
49. Yasuda K, Saitoh Y, Okita K, Morris S, Moriwaki M, Miyagawa J. Giant intrasellar arachnoid cyst manifesting as adrenal insufficiency due to hypothalamic dysfunction-case report. Neurol Med Chir (Tokyo). 2005. 45: 164-7