- Department of Neurosurgery, Hospital Regional “1° de Octubre,” Institute of Social Security and Services for State Workers (ISSSTE), Mexico
- School of Medicine, Universidad Anahuac Veracruz Campus Xalapa, Xalapa, Mexico
- Neural Dynamics and Modulation Lab, Cleveland Clinic, Cleveland, United States
Correspondence Address:
Gerardo Marin, Neural Dynamics and Modulation Lab, Cleveland Clinic, Cleveland, United States.
DOI:10.25259/SNI_657_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Carlos Castillo-Rangel1, Jose de J. Gutierrez-Banos1, Mauricio Rodriguez-Pereira1, Jaime Ordonez-Granja1, Helen Ruvalcaba-Guerrero2, Gerardo Marin3. Endovascular embolization combined with anterior cervical corpectomy for treatment of cervical spinal dural arteriovenous fistula. 20-Sep-2024;15:341
How to cite this URL: Carlos Castillo-Rangel1, Jose de J. Gutierrez-Banos1, Mauricio Rodriguez-Pereira1, Jaime Ordonez-Granja1, Helen Ruvalcaba-Guerrero2, Gerardo Marin3. Endovascular embolization combined with anterior cervical corpectomy for treatment of cervical spinal dural arteriovenous fistula. 20-Sep-2024;15:341. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13105
Abstract
Background: The two main treatments for spinal dural arteriovenous fistula (SDAVF) include microsurgical occlusion or endovascular embolization (i.e., the latter alone has high recurrence rates). Here, we combined both strategies to treat/obliterate a cervical SDAVF more effectively.
Case Description: A 34-year-old male presented with a marked decline in mental status attributed to an infratentorial subarachnoid hemorrhage. The left vertebral angiogram revealed a ruptured, low cervical SDAVF. He underwent successful occlusion of the spinal fistula utilizing super selective catheterization and endovascular embolization (i.e., utilizing Onyx-18 for the obliteration of target arteries). Due to significant SDAVF accompanying vessel recruitment/complex angioarchitecture, we additionally performed a C5 anterior corpectomy/fusion to afford direct access and complete surgical SDAVF occlusion. Three and 6 months later, repeated angiograms confirmed no recurrent or residual SDAVF.
Conclusion: We successfully treated a low cervical SDAVF using a combination of endovascular embolization and direct surgical occlusion through an anterior C5 corpectomy with a fusion approach.
Keywords: Anterior cervical approach, Corpectomy, Endovascular embolization, Hybrid strategy, Spinal dural arteriovenous fistula
INTRODUCTION
Spinal dural arteriovenous fistulas (SDAVFs) consist of abnormal connections between meningeal arteries and dural venous sinuses or subarachnoid veins that lack a capillary network.[
Direct microsurgical occlusion of these fistulas is the gold standard and has the highest success rates.[
CASE DESCRIPTION
For 6 days, a 34-year-old male experienced a mild headache, irritability, cervical/dorsal pain, and numbness of the upper extremities. He then acutely became obtunded, requiring intubation, sedation, and hospital transfer. The non-contrast cranial computed tomography scan revealed an infratentorial subarachnoid hemorrhage [
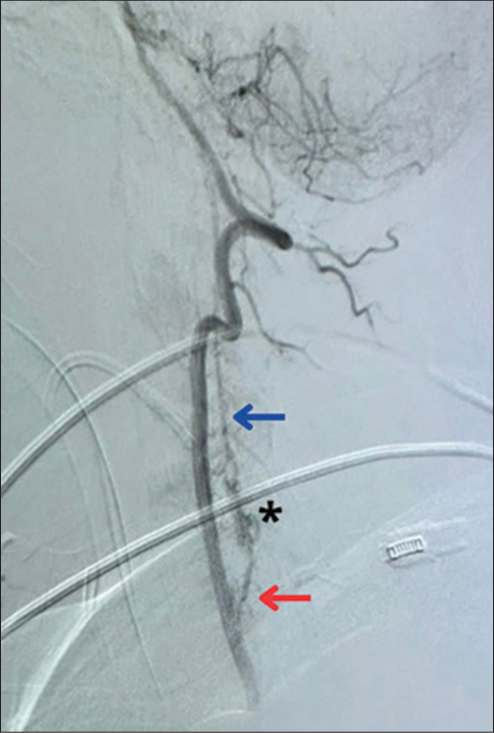
1st endovascular embolization of the left C6 radicular artery
The patient first underwent endovascular embolization of the left C6 radicular artery utilizing Onyx-18. However, the SDAVF was also being supplied through the right C5 radicular artery, with retrograde flow to the left C6 radicular artery, persistent retrograde drainage to the anterior spinal venous plexus, and an intranidal aneurysm [
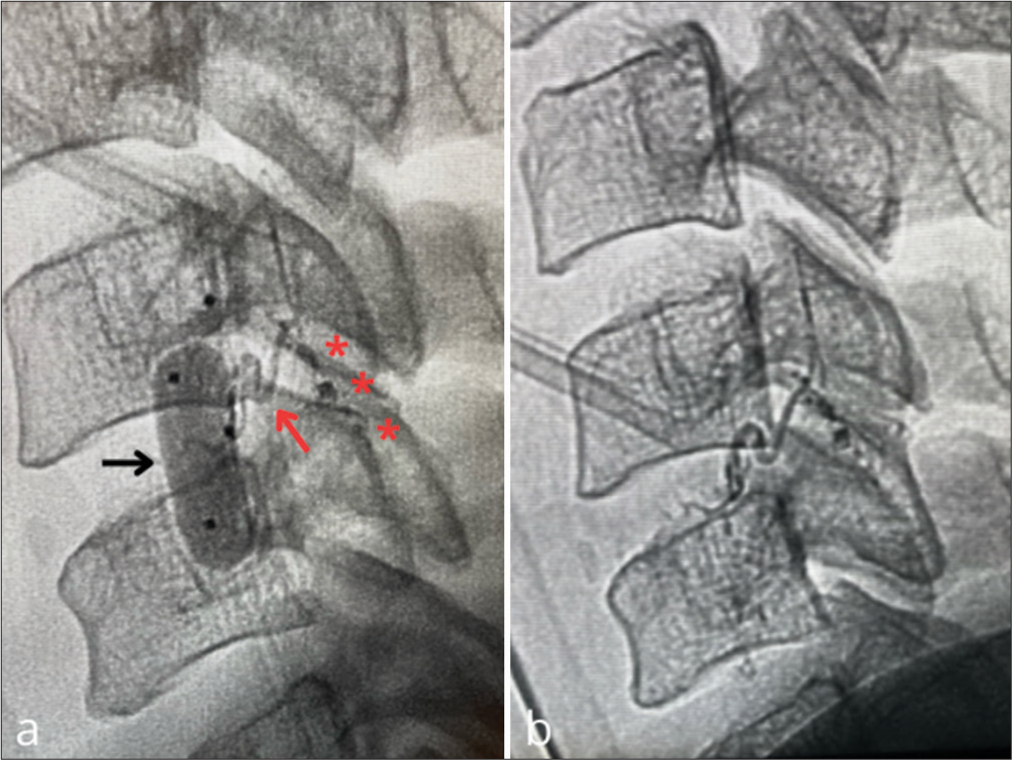
Super selective catheterization of the right C5 radicular artery
During the same procedure, super selective catheterization of the right C5 radicular artery was performed through the right vertebral artery (i.e., a microcatheter with a microwire was accessed approximately 2 cm in front of the ostium of the right C5 radicular artery). A 4 × 20 mm extra-compliant balloon was placed in the V2 segment of the right vertebral artery to keep the microcatheter in place and prevent the reflux of the embolic agent [
Figure 4:
Super selective catheterization of the right C5 radicular artery. (a) shows the balloon (black arrow) at the V2 segment of the right vertebral artery and the microcatheter placed in the target artery (red arrow); casts of Onyx-18 are seen (red asterisks). (b) right vertebral artery angiogram confirmed obliteration of the fistula.
Complete surgical occlusion of the spinal fistula through a C5 corpectomy
The preoperative embolization facilitated direct visualization and occlusion of the spinal fistula and intranidal aneurysm through an anterior C5 corpectomy/fusion (i.e., including placement of an interbody spacer and lordotic plate) [
Postoperative course
Postoperatively, the patient had 3+/5 strength in the right upper extremity. Angiograms repeated 3 and 6 months later confirmed complete occlusion of the SDAVF, and the patient will remain under angiographic surveillance every year, looking for SDAVF recurrence.
DISCUSSION
Natural history of SDAVF
Spinal fistulas typically enlarge over time.[
Efficacy of direct surgical occlusion of SDAVF
One meta-analysis quoted a 98% success rate for direct surgical occlusion of SDAVF versus a much lower 46% rate of success with embolization.[
Potential benefits of the hybrid approach to SDAVF
Few studies in the literature utilized both endovascular embolization and simultaneous direct surgical occlusion of SDAVF [
CONCLUSION
We successfully treated a low cervical SDAVF in a 34-year-old male utilizing both preoperative embolization and direct surgical occlusion through an anterior C5 corpectomy approach.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alkhaibary A, Alharbi A, Alnefaie N, Alammar H, Arishy AM, Alghanim N. Spinal dural arteriovenous fistula: A comprehensive review of the history, classification systems, management, and prognosis. Chin Neurosurg J. 2024. 10: 2
2. Cenzato M, Debernardi A, Stefini R, D’Aliberti G, Piparo M, Talamonti G. Spinal dural arteriovenous fistulas: Outcome and prognostic factors. Neurosurg Focus. 2012. 32: E11
3. El Naamani K, Tjoumakaris SI, Gooch MR, Jabbour P. Dural arteriovenous fistula. Neurosurg Clin N Am. 2024. 35: 331-42
4. Flores BC, Klinger DR, White JA, Batjer HH. Spinal vascular malformations: Treatment strategies and outcome. Neurosurg Rev. 2017. 40: 15-28
5. Koch MJ, Stapleton CJ, Agarwalla PK, Torok C, Shin JH, Coumans J. Open and endovascular treatment of spinal dural arteriovenous fistulas: A 10-year experience. J Neurosurg Spine. 2017. 26: 519-23
6. Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009. 30: 639-48
7. Lee HS, Kang HS, Kim SM, Kim CH, Yang SH, Han MH. Treatment strategy to maximize the treatment outcome of spinal dural arteriovenous fistula after initial endovascular embolization attempt at diagnostic angiography. Sci Rep. 2021. 11: 10004
8. Oh Y, Heo Y, Jeon SR, Roh SW, Park JH. Microsurgery versus endovascular treatment-which is adequate for initial treatment of spinal dural arteriovenous fistula: A case series. Neurospine. 2021. 18: 344-54
9. Oldfield EH, Di Chiro G, Quindlen EA, Rieth KG, Doppman JL. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg. 1983. 59: 1019-30
10. Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single-institution series and meta-analysis. Neurosurgery. 2004. 55: 77-87
11. Xiao Z, Gao W, Zhou H, Zhang X, Dai J, Wan J. Clinical features, angio-architectural phenotypes, and treatment strategy of foramen magnum dural arteriovenous fistulas: A retrospective case series study. Front Neurol. 2023. 14: 1121075