- Department of Neurosurgery, All India Institute of Medical Sciences, Patna, Bihar, India.
Correspondence Address:
Vikas Chandra Jha, Department of Neurosurgery, All India Institute of Medical Sciences, Patna, Bihar, India.
DOI:10.25259/SNI_310_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Vikas Chandra Jha, Mohammad Shahnawaz Alam, Vivek Sharan Sinha, Gaurav Verma, Nitish Kumar, Rahul Jain. Endovascular glue embolization of the medial posterior choroidal artery aneurysm: A case report and a literature review. 20-Oct-2023;14:370
How to cite this URL: Vikas Chandra Jha, Mohammad Shahnawaz Alam, Vivek Sharan Sinha, Gaurav Verma, Nitish Kumar, Rahul Jain. Endovascular glue embolization of the medial posterior choroidal artery aneurysm: A case report and a literature review. 20-Oct-2023;14:370. Available from: https://surgicalneurologyint.com/surgicalint-articles/12600/
Abstract
Background: The medial posterior choroidal artery (MPCA) aneurysm is extremely uncommon. Thus yet, just a few cases have been reported. Due to the deep position, narrow lumen, fragile walls, and extensive tortuosity, both endovascular and microsurgical procedures are strictly limited. A case study of successful endovascular glue embolization of a left MPCA aneurysm and a literature review is included in this report.
Case Description: A 17-year-old female arrived at our institution 2 days after suffering a major intraventricular hemorrhage with a minor subarachnoid hemorrhage. Digital subtraction angiography revealed a left MPCA aneurysm. The patient underwent a successful endovascular glue embolization and had a favorable functional outcome.
Conclusion: Endovascular glue embolization yielded favorable clinical and angiographic results in MPCA aneurysms where microcatheter access and maneuverability are challenging.
Keywords: Choroidal artery, Endovascular glue embolization, Intraventricular hemorrhage, Medial posterior choroidal artery aneurysm, Subarachnoid hemorrhage
INTRODUCTION
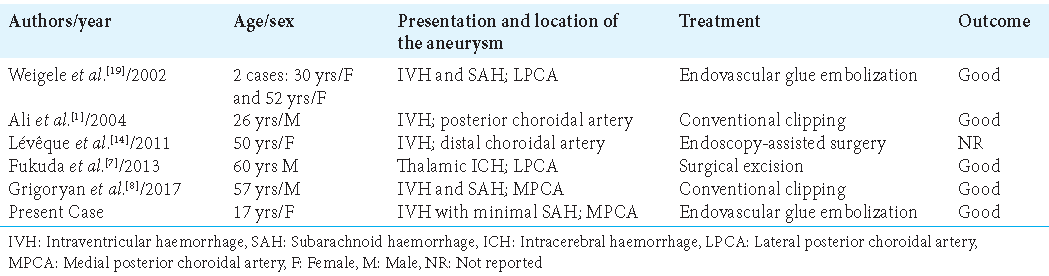
Intraventricular or distal aneurysms are uncommon diseases, with most occurrences being related to moyamoya disease or being idiopathic. Indeed, 22 cases of posterior choroidal artery aneurysms have been reported in clinical practice to date, 16 of which are associated with moyamoya, four of which are idiopathic, and two of which are associated with arteriovenous malformation.[
Medial posterior choroidal artery (MPCA) aneurysms are rare in clinical practice, having been diagnosed in just five cases;[
Lateral posterior choroidal artery (LPCA) aneurysms are more prevalent, with over 20 examples documented in the literature.[
CASE PRESENTATION
A 17-year-old female presented with a sudden onset severe headache followed by a loss of consciousness for the past 2 days. She had left blurring of vision and weakness in the left half of her face for the past 2 days. The patient had no history of seizures or systemic infections. There was no history of substance abuse or any history favoring coagulation disorders. No similar history was present in any of the family members. Normal developmental milestones were met by the patient. On examination, Glasgow Coma Scale was E3V4M5, pulse was 78/min, and blood pressure was 112/72 mmHg. There was no neck rigidity. The tone and deep tendon reflexes were normal. The plantar was an extensor on the left side. Cranial nerve examination revealed the left homonymous hemianopia and upper motor neuron type of the left facial paresis (House-Brackmann grade 2).
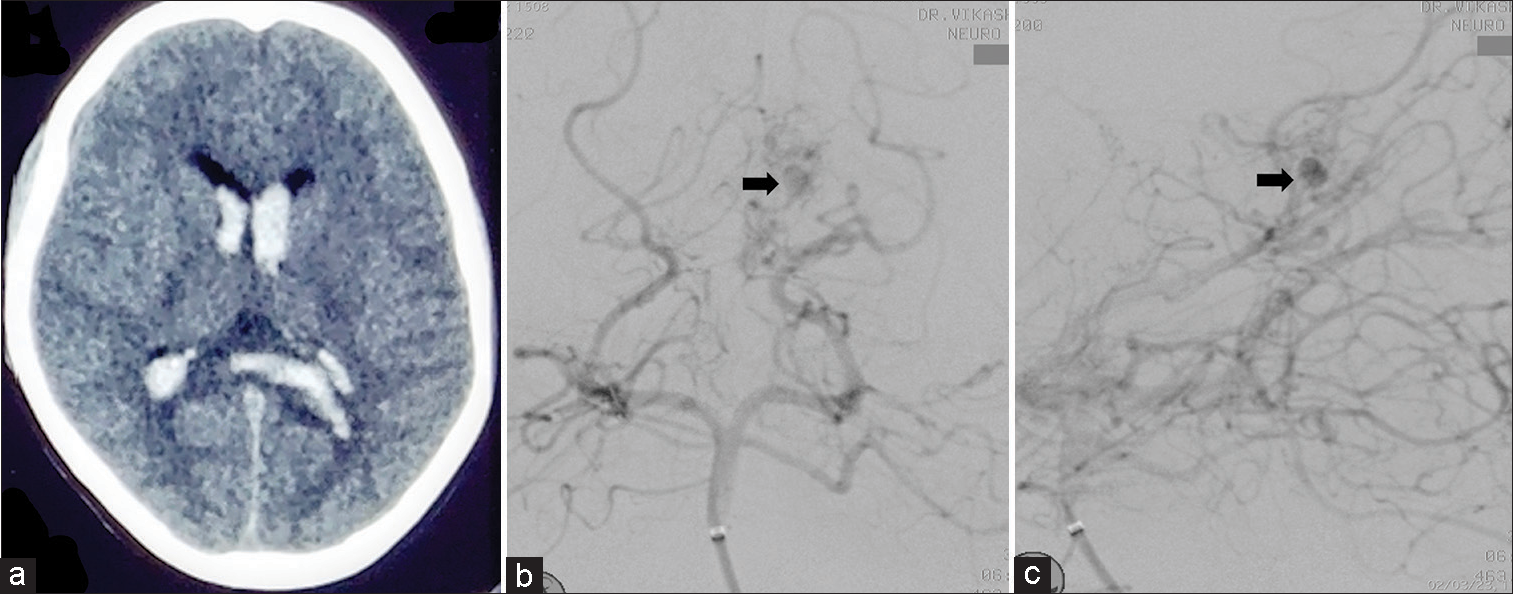
A non-contrast computed tomography (CT) scan of the brain revealed a large acute intraventricular with minimal subarachnoid hemorrhage [
Figure 1:
Initial presentation. (a) A non-contrast computed tomography scan of the brain revealed a large acute intraventricular hemorrhage almost filling all the horns of the ventricles with minimal subarachnoid hemorrhage. (b) Anteroposterior and (c) lateral projection digital subtraction angiography after right vertebral artery injection showing distally placed aneurysm arise from the left medial posterior choroidal artery which is directed upward and medially (arrow).
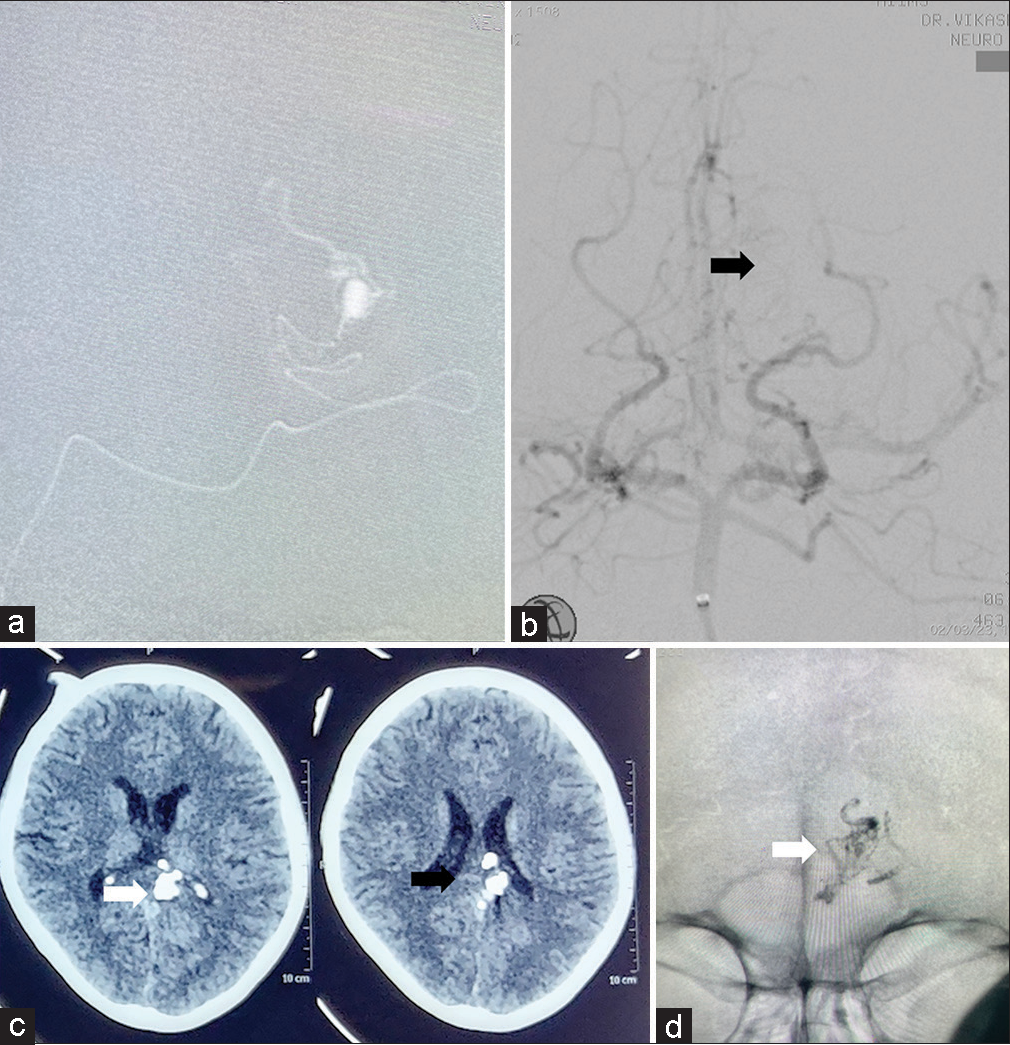
Due to its distal location in the atrium below the splenium, the microsurgical approach may have increased the patient’s visual problem and it was technically difficult to localize the aneurysm. Microsurgical dissection may also have more chances of intraoperative rupture so we planned for endovascular embolization. Under general anesthesia, femoral artery access was achieved. For distal access, we used a 5F long femoral sheath (Cook Medical, Bloomington, IN, USA), NavienTM Intracranial Support Catheter (Covidien Vascular Therapies, Mansfield, MA, USA), MarathonTM microcatheter (010) (Medtronic, Minneapolis, Minnesota, USA), and MirageTM microguidewire, (008) (EV3, Mansfield, MA, USA). Moreover, we performed super-selective angiography to look for the angioarchitecture of the aneurysm and the parent vessel [
Figure 2:
Endovascular Glue embolization. (a) Super-selective digital subtraction angiography (DSA) showing the tortuosity of the parent vessel and the angioarchitecture of the distally placed aneurysm arising from the left medial posterior choroidal artery. (b) Check DSA showing the complete obliteration of the aneurysm with maintained parent vessels and distal flow (black arrow). Postprocedure (c), non-contrast computed tomography head, and (d), skull fluoroscopy showing the glue cast (white arrow).
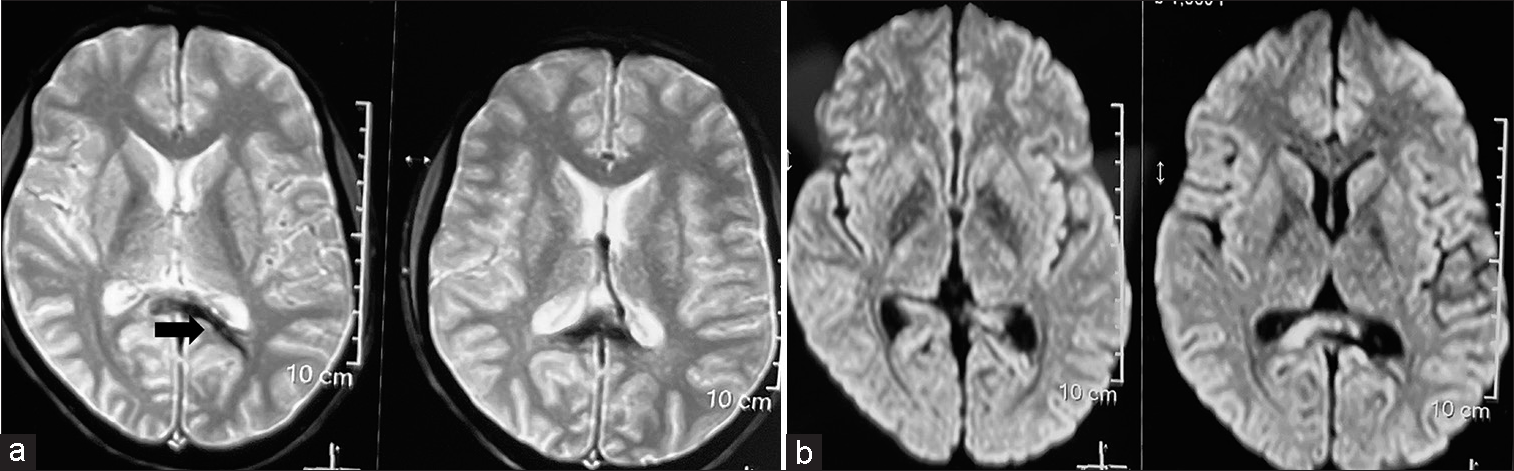
Figure 3:
Postoperative magnetic resonance imaging. (a) Axial view susceptibility weighted image showing abnormal blooming (arrow) at the junction of the trigone and left occipital horn of the lateral ventricle likely depicting the glue cast. (b) Diffusion-weighted image showing no evidence of infarct or any abnormal diffusion restriction.
DISCUSSION
The posterior cerebral artery (PCA) gives rise to the medial and lateral posterior choroidal arteries. In 14.3% of cases, the MPCA arises from the P1 segment of the PCA; in 70% of cases, the artery branches into a single vessel at the beginning of the P2 segment; and much less frequently, the MPCA arises from the distal segment of the PCA. The MPCA envelops the lateral surface of the midbrain and supplies the pineal gland, medial and dorsal sections of the pulvinar, superior colliculus of the quadrigeminal, lateral and medial geniculate bodies, brain stem, tegmentum, and medial and lateral geniculate bodies through a number of small perforators.[
Fusiform aneurysms of the terminal segments of the choroidal arteries are known as intraventricular aneurysms or peripheral aneurysms. They sometimes have no visible neck. Due to their tiny size, deep position, and anatomical structural characteristics, posterior choroidal artery aneurysms are difficult to cure surgically or endovascularly. Few papers specifically address the endovascular treatment of MPCA aneurysms, highlighting its complexity and the difficulties of selective exclusion of the aneurysm.[
A few studies have documented spontaneous regression of distal choroidal artery aneurysms after 6 weeks–11 months.[
CONCLUSION
Symptomatic MPCA aneurysms are highly uncommon and typically present with severe headaches and massive intraventricular hemorrhage. Endovascular glue embolization may be an appealing option since it avoids the complications of craniotomy and dissection while providing superior clinical outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ali MJ, Bendok BR, Getch CC, Gottardi-Littell NR, Mindea S, Batjer HH. Surgical management of a ruptured posterior choroidal intraventricular aneurysm associated with moyamoya disease using frameless stereotaxy: Case report and review of the literature. Neurosurgery. 2004. 54: 1019-24
2. Alshumrani GA, Al-Qahtani S. Endovascular embolization of a giant aneurysm in medial posterior choroidal artery with associated arteriovenous malformation. Ann Saudi Med. 2013. 33: 307-9
3. Baik SK, Kim YS, Lee HJ, Kim IS, Kang DS. Endovascular management of peripheral lateral posterior choroidal artery aneurysm associated with moyamoya disease: Case report and review of the literature. Neurointervention. 2006. 1: 44-9
4. Calle JL, Zuazo RA, Valverde RP, Cáceres WC, Barboza KC, Torres CF. Clipping of lateral posterior choroidal intraventricular aneurysm related to ruptured temporal arteriovenous malformation: A case report. Open J Mod Neurosurg. 2016. 6: 9-15
5. Chen CJ, Caruso J, Starke RM, Ding D, Buell T, Crowley RW. Endoport-assisted microsurgical treatment of a ruptured periventricular aneurysm. Case Rep Neurol Med. 2016. 2016: 8654262
6. Fujii K, Lenkey C, Rhoton AL. Microsurgical anatomy of the choroidal arteries: Lateral and third ventricles. J Neurosurg. 1980. 52: 165-88
7. Fukuda H, Munoz D, Macdonald RL. Spontaneous thalamic hemorrhage from a lateral posterior choroidal artery aneurysm. World Neurosurg. 2013. 80: 900.e1-6
8. Grigoryan YA, Sitnikov AR, Timoshenkov AV, Grigoryan GY. An aneurysm of the medial posterior choroidal artery: A case report and a literature review. Zh Vopr Neirokhir Im N N Burdenko. 2017. 81: 101-7
9. He K, Zhu W, Chen L, Mao Y. Management of distal choroidal artery aneurysms in patients with moyamoya disease: Report of three cases and review of the literature. World J Surg Oncol. 2013. 11: 187
10. Kheireddin AS, Filatov YM, Shishkina LV, Shitov AM, Okishev DN, Baranov OA. Aneurysm of posterior lateral choroid artery. Zh Vopr Neirokhir Im N N Burdenko. 2010. 4: 52-4
11. Kim SH, Kwon OK, Jung CK, Kang HS, Oh CW, Han MH. Endovascular treatment of ruptured aneurysms or pseudoaneurysms on the collateral vessels in patients with moyamoya disease. Neurosurgery. 2009. 65: 1000-4
12. Kolotvinov VS, Sakovich VP, Shamov AY, Strakhov AA, Astashov AS, Marchenko OV. Surgical treatment of female patient with aneurysm of posterior choroid artery in acute period of hemorrhage. Russ J Neurosurg. 2013. 2: 95-7
13. Kwak R, Yamamoto N, Ito S, Kadoya S. A case of moyamoya disease associated with a peripheral artery aneurysm of the thalamus. No Shinkei Geka. 1984. 12: 1419-23
14. Lévêque M, McLaughlin N, Laroche M, Bojanowski MW. Endoscopic treatment of distal choroidal artery aneurysm. J Neurosurg. 2011. 114: 116-9
15. Miyake H, Ohta T, Kajimoto Y, Ogawa R, Deguchi J. Intraventricular aneurysms--three case reports. Neurol Med Chir (Tokyo). 2000. 40: 55-60
16. Ohta T, Ozawa H, Yamauchi T, Kubokura T. Ruptured aneurysm of the medial posterior choroidal artery first demonstrated by the microcatheter technique-case report. Neurol Med-Chir (Tokyo). 2003. 43: 601-4
17. Okawa M, Abe H, Ueba T, Higashi T, Inoue T. Surgical management of a ruptured posterior choroidal intraventricular aneurysm associated with moyamoya disease. Open Med. 2013. 8: 818-21
18. Ungersböck K, Perneczky A. Intraventricular aneurysm of the medial posterior choroid artery clipped via the contralateral transcallosal approach. Acta Neurochir. 1986. 82: 24-7
19. Weigele JB, Chaloupka JC, Lesley WS, Mangla S, Hitchon PW VanGilder JC. Peripheral aneurysms of the lateral posterior choroidal artery: Clinical presentation and endovascular treatment: Report of two cases. Neurosurgery. 2002. 50: 392-6
20. Yamada H, Saga I, Kojima A, Horiguchi T. Short-term spontaneous resolution of ruptured peripheral aneurysm in moyamoya disease. World Neurosurg. 2019. 126: 247-51
21. Yang S, Yu JL, Wang HL, Wang B, Luo Q. Endovascular embolization of distal anterior choroidal artery aneurysms associated with moyamoya disease. A report of two cases and a literature review. Interv Neuroradiol. 2010. 16: 433-41
22. Yuan Z, Woha Z, Weiming X. Intraventricular aneurysms: Case reports and review of the literature. Clin Neurol Neurosurg. 2013. 115: 57-64