- Department of Neurosurgery, Nara Medical University, Kashihara, Japan.
Correspondence Address:
Ichiro Nakagawa, Department of Neurosurgery, Nara Medical University, Kashihara, Japan.
DOI:10.25259/SNI_515_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ai Okamoto, Ichiro Nakagawa, Masashi Kotsugi, Shohei Yokoyama, Shuichi Yamada, Young-Soo Park, Hiroyuki Nakase. Endovascular vertebral artery orifice angioplasty for the prevention of acute ischemic stroke following vertebral artery stump syndrome. 26-Aug-2022;13:382
How to cite this URL: Ai Okamoto, Ichiro Nakagawa, Masashi Kotsugi, Shohei Yokoyama, Shuichi Yamada, Young-Soo Park, Hiroyuki Nakase. Endovascular vertebral artery orifice angioplasty for the prevention of acute ischemic stroke following vertebral artery stump syndrome. 26-Aug-2022;13:382. Available from: https://surgicalneurologyint.com/surgicalint-articles/11829/
Abstract
Background: Vertebral artery stump syndrome (VASS) involves repeated acute ischemic stroke (AIS) in the posterior circulation following vertebral artery (VA) orifice occlusion. The presence of VA orifice occlusion makes endovascular thrombectomy (EVT) difficult to achieve and leads to posterior circulation stroke with unfavorable functional outcomes. Here, we report a case of endovascular VA orifice angioplasty for the right VA pseudo-occlusion to prevent AIS following VASS pathology.
Case Description: In a 76-year-old man presenting with dizziness, angiography revealed right pseudo-occluded VA at the origin concomitant with the left VA occlusion. The posterior circulation depended on the right VA through collateral flow to the distal portion. Prophylactic endovascular VA angioplasty for the right pseudo-occluded VA at the orifice was achieved to prevent AIS with tandem lesions. In the present case, endovascular VA angioplasty can prevent acute embolic stroke in the posterior circulation following EVT-resistant VASS pathology.
Conclusion: Clinicians should be aware that EVT is not easy in AIS following VASS due to access difficulties and the treatment strategy should be carefully considered.
Keywords: Acute ischemic stroke, Endovascular angioplasty, Mechanical thrombectomy, Posterior circulation, Vertebral artery stump syndrome
INTRODUCTION
Vertebral artery stump syndrome (VASS) is a pathophysiology in which embolic strokes develop in the posterior circulation due to stagnating clot fragments through collateral artery flow after vertebral artery (VA) occlusion.[
CASE REPORT
History and examination
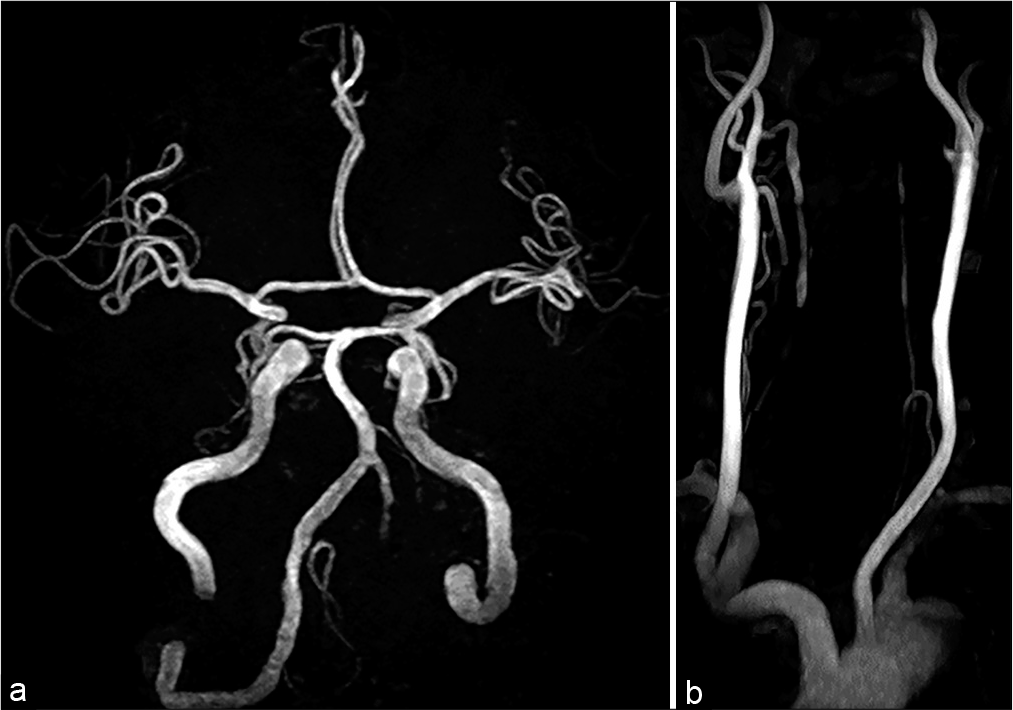
A 76-year-old man, with asymptomatic left VA stenosis noted on magnetic resonance angiography (MRA) 3 years earlier, presented with sudden dizziness. MRA showed left VA occlusion [
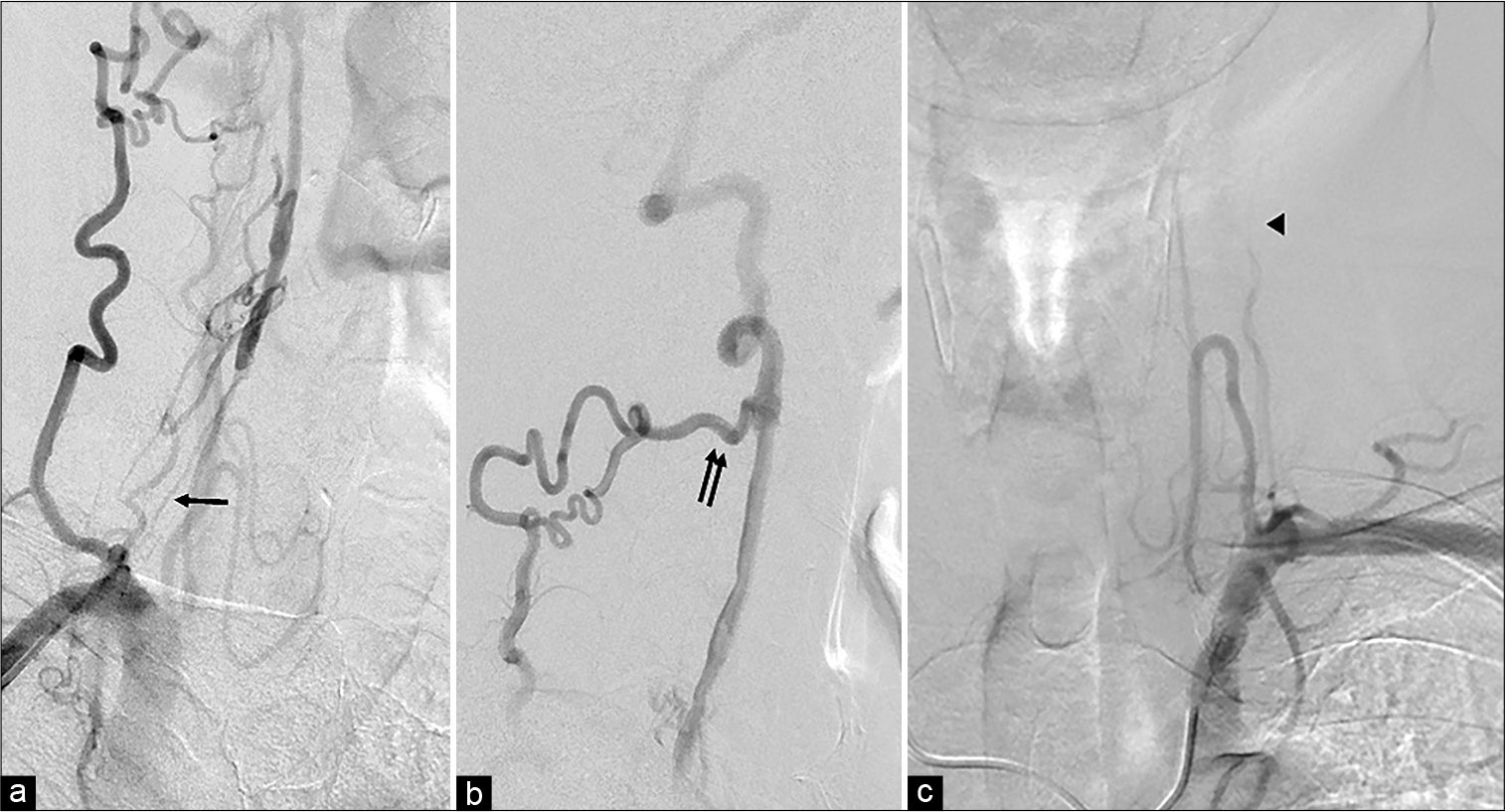
He was referred to our department and showed no neurological deficits including dizziness on examination. Digital subtraction angiography showed right VA orifice pseudo-occlusion with faint anterograde flow [
Figure 2:
(a) The right anterior oblique view of the vertebral artery (VA) angiogram shows pseudo-occlusion of the right VA orifice with faint anterograde flow (arrow). (b) Lateral view of the right VA lateral angiogram reveals that the right VA is reconstructed with a collateral pathway through the deep cervical arteries (double arrows). (c) The left subclavian angiogram reveals left VA occlusion at the C2 level (arrowhead).
Treatment
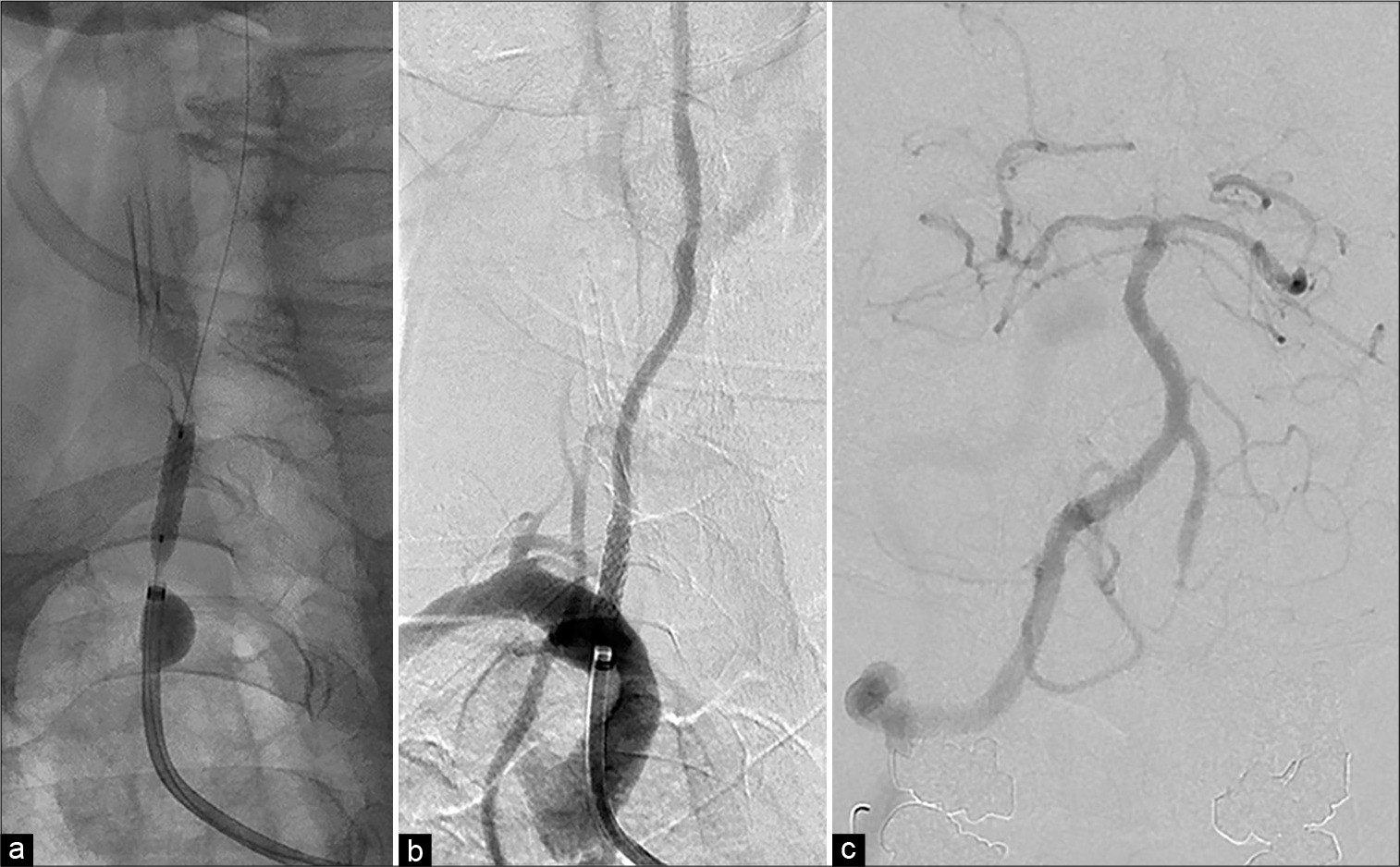
An 8-Fr OPTIMO balloon guiding catheter (Tokai Medical Products, Aichi, Japan) was positioned at the right subclavian artery near the right VA origin through right common femoral artery access under local anesthesia. A 0.014-inch microguidewire, ASAHI CHIKAI (Asahi Intecc Co. Ltd., Aichi, Japan) was passed through the pseudo-occluded VA ostium under guiding balloon inflation. Since near-infrared spectroscopy intravascular ultrasonography did not show a lipid core or mobile plaque, percutaneous transluminal angioplasty (PTA) was performed using a 2.5 mm balloon (Rapid cross: Medtronic, Minneapolis, USA) without distal protection. A balloon expandable bare-metal stent (4 mm diameter, 15 mm length, PALMAZ Genesis; Cordis, Dublin, Ireland) was placed [
Postoperative course
Postoperative diffusion-weighted imaging showed only a single, small, and bright lesion in the left cerebellar hemisphere and the right VA was visualized well on MRA. He was discharged 8 days after treatment without neurological deficits. Postoperative ultrasonography showed good stent patency and anterograde flow through the right VA orifice at both 1 week and 3 months after treatment. No onset of VASS was seen at the 1-year follow-up and MRA showed no restenosis.
DISCUSSION
We have reported successful endovascular VA orifice angioplasty for the prevention of acute embolic stroke in the posterior circulation following EVT-resistant VASS pathology.
Nguyen et al. first reported a syndrome of stroke in the posterior circulation after VA occlusion and named this phenomenon “vertebral stump syndrome” after the carotid artery stump and ongoing ischemic events despite the occluded artery.[
One of the treatments for acute cerebral infarction due to VASS is antiplatelet therapy with clopidogrel, together with intravenous infusion of ozagrel.[
In this case, emergent EVT for intracranial tandem embolic arterial occlusion following VASS may not be achievable because of difficulties reaching the lesion. We, therefore, decided to perform endovascular VA orifice angioplasty for pseudo-occluded VA at the origin not only to prevent AIS following VASS but also to secure the access route for emergent EVT. Although this treatment was intended to prevent the development of AIS following VASS, prospective studies are required to determine whether this prophylactic VA orifice angioplasty treatment will improve the development of AIS and functional prognosis in patients with VA occlusive lesions.
CONCLUSION
We report a case of endovascular VA orifice angioplasty for the prevention of AIS in the posterior circulation following VASS. Clinicians should be aware that EVT is not easy in AIS following VASS due to access difficulties, and the treatment strategy should be carefully considered.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chen L, Jiang Y, Hu F, He L, Zheng H. Endovascular revascularization of nonacute symptomatic proximal extracranial vertebral artery occlusion. World Neurosurg. 2020. 134: 39-44
2. Kawano H, Inatomi Y, Hirano T, Yonehara T, Uchino M. Anticoagulation therapy for vertebral artery stump syndrome. J Neurol Sci. 2010. 295: 125-7
3. Kawano H, Inatomi Y, Hirano T, Yonehara T. Vertebral artery stump syndrome in acute ischemic stroke. J Neurol Sci. 2013. 324: 74-9
4. Maeoka R, Nakagawa I, Ohnishi H, Kuga Y, Nakase H, Ohnishi H. A thread of hope for successful revascularization for acute embolic basilar artery occlusion due to miserable vertebral artery stump syndrome. A technical report. J Clin Neurosci. 2020. 73: 299-303
5. Moufarrij NA, Little JR, Furlan AJ, Williams G, Marzewski DJ. Vertebral artery stenosis: Long-term follow-up. Stroke. 1984. 15: 260-3
6. Nguyen TN, Raymond J, Mahmoud M, Weill A, Roy D, Guilbert F. Vertebral artery stump syndrome. J Neurol Neurosurg Psychiatry. 2008. 79: 91-2
7. Nii K, Abe G, Iko M, Nomoto Y, Yu I, Sakamoto K. Endovascular angioplasty for extracranial vertebral artery occlusion without visualization of the stump of the artery ostium. Neurol Med Chir (Tokyo). 2013. 53: 422-6
8. Suzuki M, Dembo T, Hara W, Tajima T, Yamashita M, Oji S. Vertebral artery stump syndrome. Intern Med. 2018. 57: 733-6