- Department of Neurosurgery, National University of Mexico Hospital General, Durango,
- Neurosurgery Service, Centenario Hospital Miguel Hidalgo, Aguascalientes, Mexico,
- Department of Neurosurgery, Hospital Padilla de Tucuman, Tucuman, San Miguel de Tucuman, Argentina,
- Institute of Neurosciences, Krishna Vishwa Vidyapeeth, Karad, Maharashtra, India,
- Department of Neurosurgery, Federal Center of Neurosurgery, Tyumen,
- Department of Neurosurgery, Peoples’ Friendship University of Russia, Moscow, Russian Federation,
- Department of Neurosurgery, The Royal Melbourne Hospital, Melbourne, Australia,
- Department of Neurosurgery, RUDN University, Moscow, Russian Federation,
- Neurosurgery Service, Hospital Star Medica, Chihuahua, Mexico,
- 0Spine Surgery Unit, Department of Neurosurgery, Hospital del Mar, Barcelona, Spain,
- 1Department of Neurosurgery, San Fernando Hospital, Belgrano, San Fernando, Argentina,
- 2Department of Neurosurgery, University of Pavia, Pavia,
- 3Department of Neurosurgery, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy.
Correspondence Address:
Nicola Montemurro, Department of Neurosurgery, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy.
DOI:10.25259/SNI_791_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Carlos Salvador Ovalle Torres1, Alfredo Espinosa Mora2, Alvaro Campero3, Iype Cherian4, Albert Sufianov5, Edgar Fragoza Sanchez1, Manuel Encarnacion Ramirez6, Issael Ramirez Pena7, Renat Nurmukhametov8, Macario Arellano Beltrán1, Eduardo Diaz Juarez1, Arturo Muñoz Cobos9, Jesus Lafuente-Baraza10, Matias Baldoncini11, Sabino Luzzi12, Nicola Montemurro13. Enhancing microsurgical skills in neurosurgery residents of low-income countries: A comprehensive guide. 22-Dec-2023;14:437
How to cite this URL: Carlos Salvador Ovalle Torres1, Alfredo Espinosa Mora2, Alvaro Campero3, Iype Cherian4, Albert Sufianov5, Edgar Fragoza Sanchez1, Manuel Encarnacion Ramirez6, Issael Ramirez Pena7, Renat Nurmukhametov8, Macario Arellano Beltrán1, Eduardo Diaz Juarez1, Arturo Muñoz Cobos9, Jesus Lafuente-Baraza10, Matias Baldoncini11, Sabino Luzzi12, Nicola Montemurro13. Enhancing microsurgical skills in neurosurgery residents of low-income countries: A comprehensive guide. 22-Dec-2023;14:437. Available from: https://surgicalneurologyint.com/surgicalint-articles/12684/
Abstract
Background: The main objectives of this paper are to outline the essential tools, instruments, and equipment needed to set up a functional microsurgery laboratory that is affordable for low-income hospitals and to identify cost-effective alternatives for acquiring microsurgical equipment, such as refurbished or donated instruments, collaborating with medical device manufacturers for discounted rates, or exploring local suppliers.
Methods: Step-by-step instructions were provided on setting up the microsurgery laboratory, including recommendations for the layout, ergonomic considerations, lighting, and sterilization processes while ensuring cost-effectiveness, as well as comprehensive training protocols and a curriculum specifically tailored to enhance microsurgical skills in neurosurgery residents.
Results: We explored cost-effective options for obtaining microsurgery simulators and utilizing open-source or low-cost virtual training platforms. We also included guidelines for regular equipment maintenance, instrument sterilization, and establishing protocols for infection control to ensure a safe and hygienic learning environment. To foster collaboration between low-income hospitals and external organizations or institutions that can provide support, resources, or mentorship, this paper shows strategies for networking, knowledge exchange, and establishing partnerships to enhance microsurgical training opportunities further. We evaluated the impact and effectiveness of the low-cost microsurgery laboratory by assessing the impact and effectiveness of the established microsurgery laboratory in improving the microsurgical skills of neurosurgery residents. About microsutures and microanastomosis, after three weeks of training, residents showed improvement in “surgical time” for ten separate simple stitches (30.06 vs. 8.65 min) and ten continuous single stitches (19.84 vs. 6.51 min). Similarly, there was an increase in the “good quality” of the stitches and the suture pattern from 36.36% to 63.63%.
Conclusion: By achieving these objectives, this guide aims to empower low-income hospitals and neurosurgery residents with the necessary resources and knowledge to establish and operate an affordable microsurgery laboratory, ultimately enhancing the quality of microsurgical training and patient care in low-income countries.
Keywords: Low-income countries, Microsurgery laboratory, Microsurgical skills, Neurosurgery
INTRODUCTION
Microsurgery plays a crucial role in neurosurgery, enabling intricate procedures with precision and minimizing damage to surrounding tissues. It is an essential skill that neurosurgery residents must acquire during their training (ideally after a long training in the laboratory) to ensure optimal patient outcomes.[
The final goal and main objective of our work are to show how to establish a basic microsurgery laboratory with the resources available, that could be easily replicable in low-resource hospitals/low-income countries; the whole process took over six months. We describe the usefulness and functionality of our laboratory by exposing some examples and the level reached in microsutures, filling techniques, microdissections, and anatomical-descriptive photographs accumulated over 16 weeks of carrying out the different practices.
There are multiple modalities of surgical training, among which stand out: (1) training in laboratory animal specimens; (2) training in cadaveric specimens, human organs, and tissues in the laboratory; (3) training in simulators of various types (Virtual reality, mannequins, 3D anatomical models, and robotic surgery); and (4) training and development of skills in the operating room, directly on the patient during the surgical act under the supervision of experienced neurosurgeons. This last modality was the traditional method for a long time and still predominates in countries of medium and low resources and most of Latin America due to the low medical-scientific development and the lack of investment from governments in the health sector for scientific research purposes, specifically microsurgical training laboratories.[
The first two modalities involve the microsurgery laboratory, of which we rule out the first modality, as explained before, since it is not possible to maintain and acquire animal specimens due to financial constraints in the vast majority of our hospitals. Microsurgical techniques have historically relied on the use of live animal models.[
Below, we present the steps for the creation of a low-cost, basic microsurgery laboratory in a second-level hospital with zero funds designated for microsurgical training (similar to the vast majority of hospitals in countries with medium and low resources), as well as the final result (our laboratory and some examples of our work) and future objectives in the medium and long term.
MATERIALS AND METHODS
Basic hospital equipment
Initially, we used easily accessible hospital and low-cost materials such as latex sheets/gloves, silicone tubes, gauze, microstructures of various calibers, probes, catheters, disposable surgical gowns, disposable surgical fields, needles, syringes, masks, and compresses. Most of this is provided by our institution through formal requests well-requisitioned and scientifically justified, in which it was emphasized that this material does not represent a significant expense compared to the outcome of improved microsurgical skills from trainees, which subsequently improves patient care prognosis, decreases hospital stay, and surgical time, which will ultimately benefit the entire hospital. Another part of the material was donated directly from the warehouses of our hospital, and the rest from the maternal and child hospital, being surplus material that was overbought, in disuse, or expired after the COVID-19 pandemic. We were also provided with containers and bags for medical waste by our institution. In almost any hospital, the acquisition of these resources can be achieved through a well-founded request with the support of the department head, as in our case.
Non-departmental economic resources
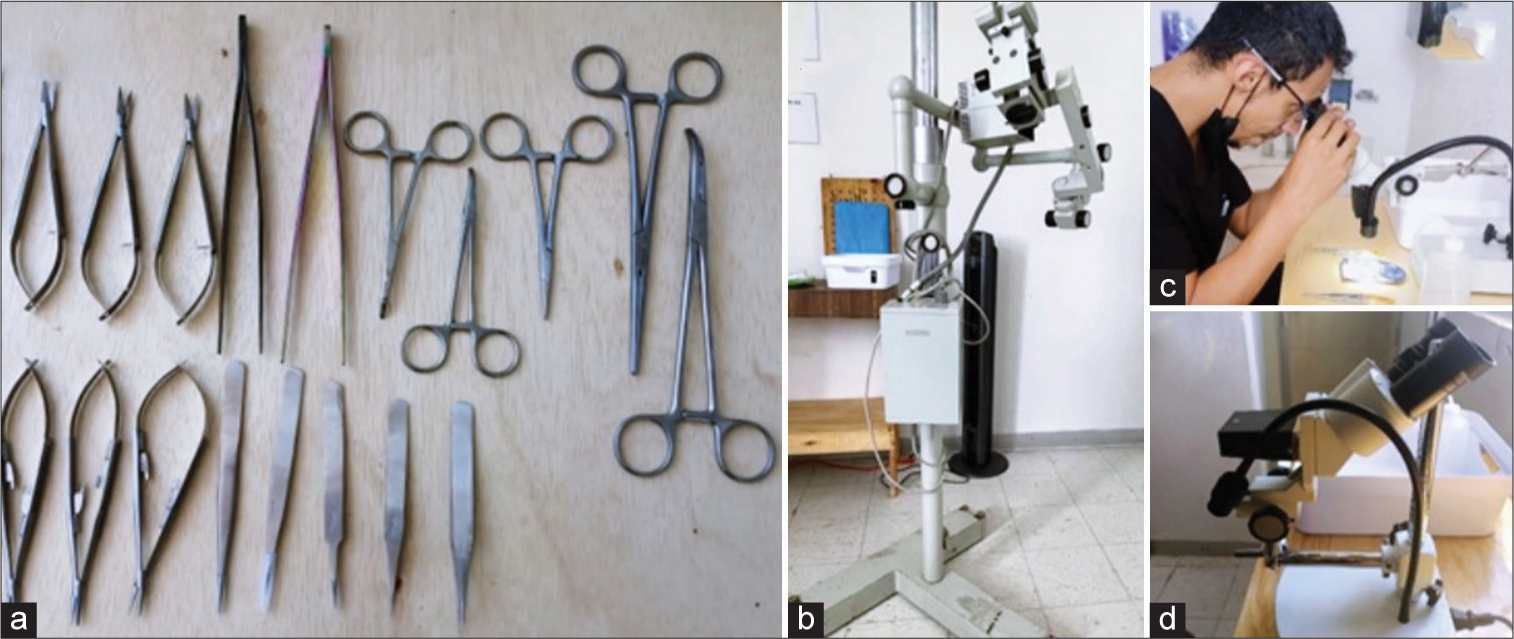
On the initiative of our group of residents, the collection of money from a part of the scholarships of each of the residents (18 residents in total), as well as some complementary economic donations by some of our most junior neurosurgeons and one of our senior neurosurgeon, by doing this, it was possible to obtain $ 1,408 that allowed the following basic material to be purchased: Carpentry custom made wood tables, a wooden bench made, low-cost high chairs from the department store, trays, plastic and storage containers, a cooler, a small second-hand refrigerator, a fan, a high-speed motorized craniotome for dental use, and basic low-cost microsurgical material which were purchased online [
Microscopes
Two old model microscopes were donated by a senior neurosurgeon, which requires an external light source for their operation; a junior neurosurgeon donated a used small benchtop stereoscopic binocular microscope with arm and light emitting diode light included and a resident donated a similar benchtop stereoscopic microscope [
Borrowed material
There is a tripod for the camera and a semi-professional camera owned by one of the residents to obtain high-quality anatomical photographs; if, in some instances, it is not available, you can perform the same process with a mobile phone of whichever has the better-quality camera, subsequently is easy to make an edition resulting in very high-quality work. In case you do not have a semi-professional camera or do not have experience in editing photographs from mobile device cameras, a plausible option would be to hire a professional photographer and make the payment through the economic collection as mentioned before or offer that the photographs will be published in journals that will contribute significantly to the development of microneurosurgery. At the moment, we have a light source and an optical fiber cable that adapts to the source, borrowed and owned by one of our senior neurosurgeons. There are also borrowed telescopic magnifiers owned by some residents on those occasions when the light source is not available. After preparing a properly requisitioned and justified request, we are waiting for a donation of two portable light sources from our institution. We know that the option of “loaning material” is not ideal and, in many cases, can be difficult to achieve; however, in our experience so far, it has worked as a complement to the operation of the laboratory.
Space for the establishment of the laboratory
One of the greatest difficulties, without a doubt, is obtaining the space to establish the laboratory; in our case, before an initial denial from the hospital authorities, we presented our project again through a more formal request that got us a small hospital room in a disused room in another hospital apart from ours, this caused the relative difficulty of moving between one hospital to another. After several months of effort, we presented our advances to our hospital authorities. Finally, we were given a large space that was, for a long time in disuse, away from the hospitalization area, located within our own neurosurgery department next to the residents’ office.
It is hard work to find a suitable space. Once found, it is equally challenging to achieve authorization from the institutions, so it is advisable, before looking for the space, to have at least the bases and foundations of the project as well as the objectives; this will help in convincing the corresponding authorities since, most of the times, these are doctors with administrative positions who are not related and unfamiliar with the subject. This becomes even more complicated when it comes to purely administrative authorities outside the medical area, who have little understanding of the importance of microsurgery. It is necessary to look for disused spaces in hospitals, which, when used for this purpose, do not affect the hospital’s workflow in any way. Ideally, it should be at least within the headquarters itself, away from the hospitalization areas, and be a prominent place with sinks (or at least a large sink), a bathroom, a good ventilation system or large windows (preferable), and a dedicated area for temporary storage, control, and management of infectious biological hazardous waste (medical waste).
Placental biological specimens
Placental specimens are easily accessible in any hospital that has an established gynecology and obstetrics service and can be obtained through local agreements with such service whenever necessary, as well as respecting the biosecurity guidelines when handling these specimens as they are medical waste.
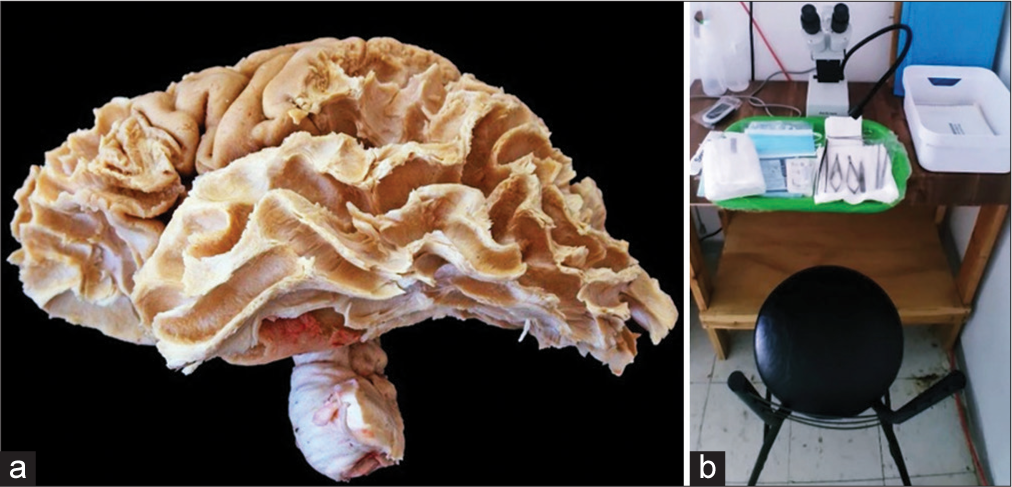
Brain specimens product of autopsy
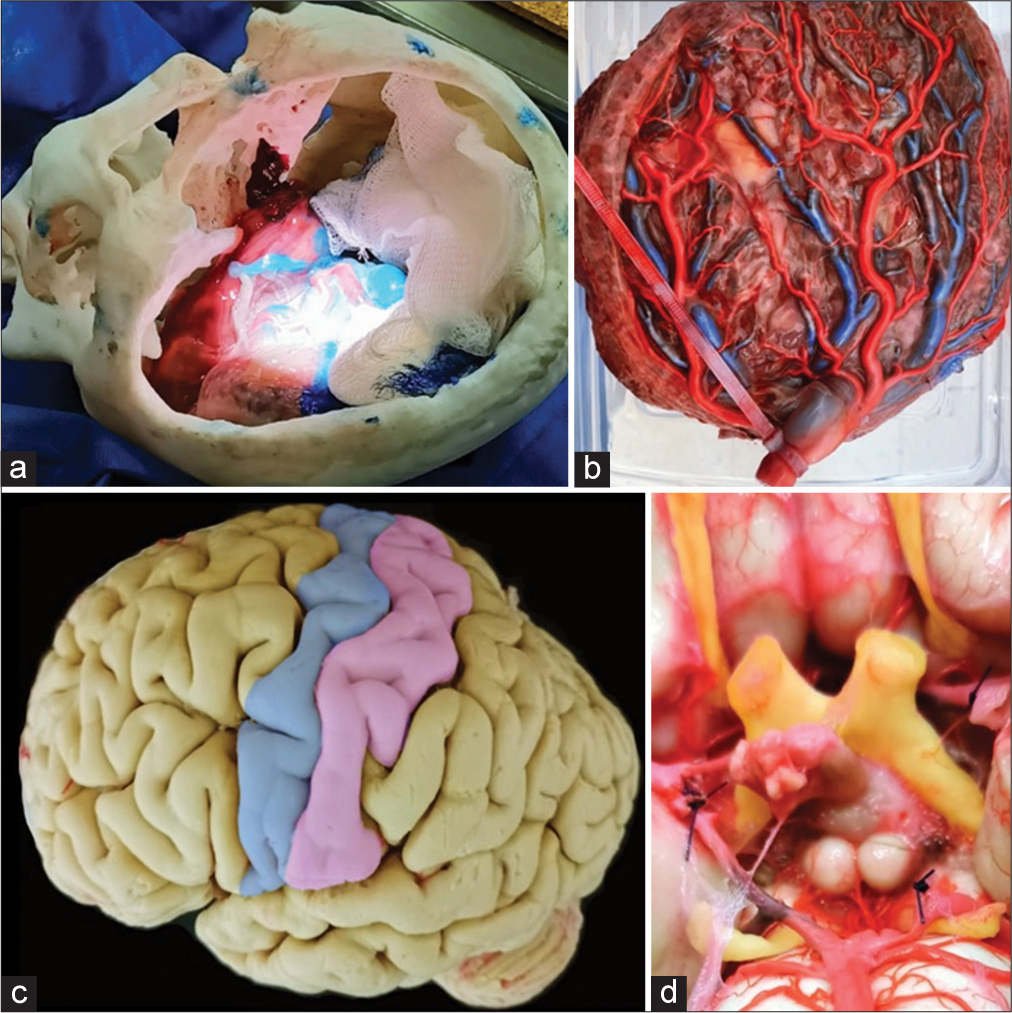
As in many hospitals, access to cadaveric specimens for study purposes can be very challenging, so we submitted a legal document to the hospital scientifically justifying the project. This legal document followed the federal health law of the country and the guidance of the pathology department regarding the proper use and disposal of organic material. The resident’s job consists of brain extraction during the autopsy, fixation process, vessel repletion, macro, and microscopic study, meticulously caring for the specimen in each phase, and the cutting and final inclusions of lamellae are done by the pathological anatomy service.[
Figure 2:
Access to cadaveric specimens for study purposes can be very challenging, so we submitted a legal document to the hospital scientifically justifying the project. This legal document followed the federal health law of the country and guidance of the pathology department regarding the proper use and disposal of organic material.
RESULTS
We took on the task of searching publications and bibliography on microsurgery; in our search, we found the “Practical Manual of Vascular and Nervous Microsurgery”[
Practices with inanimate material
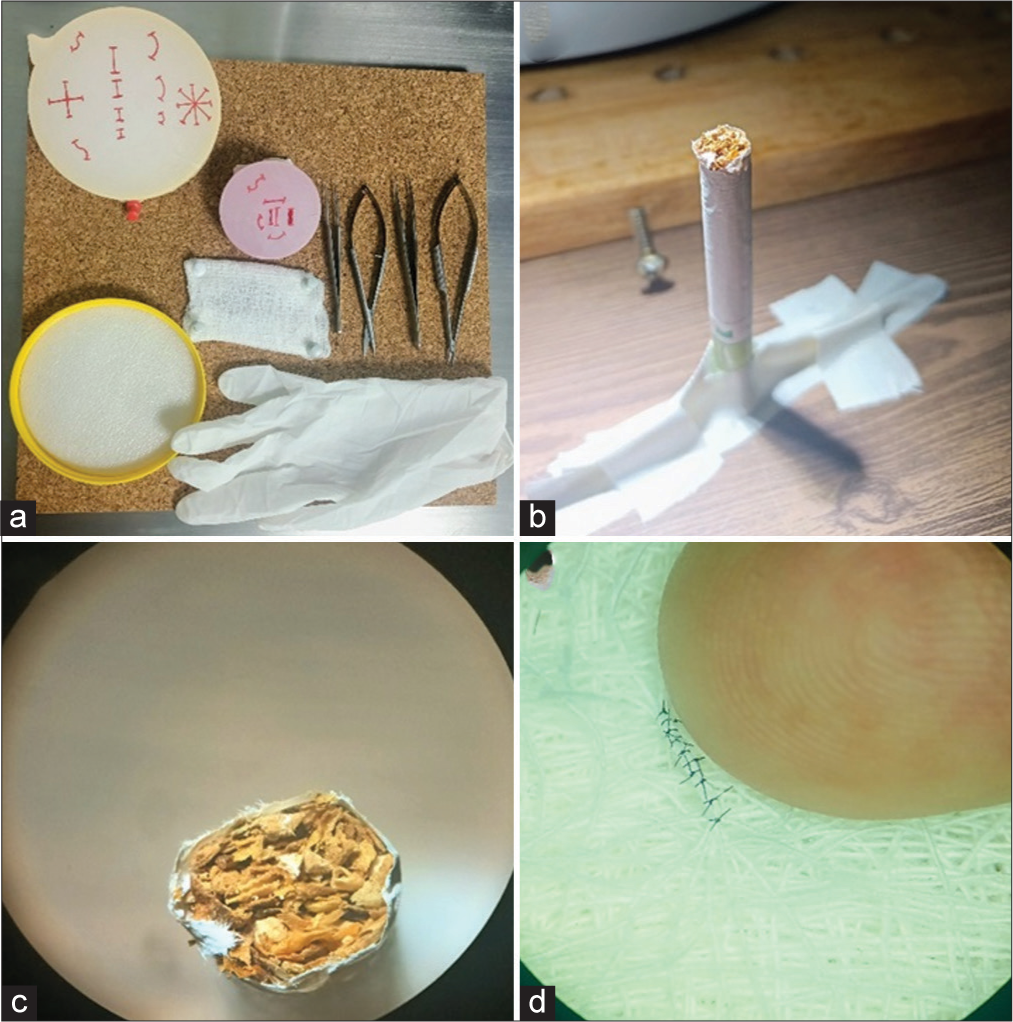
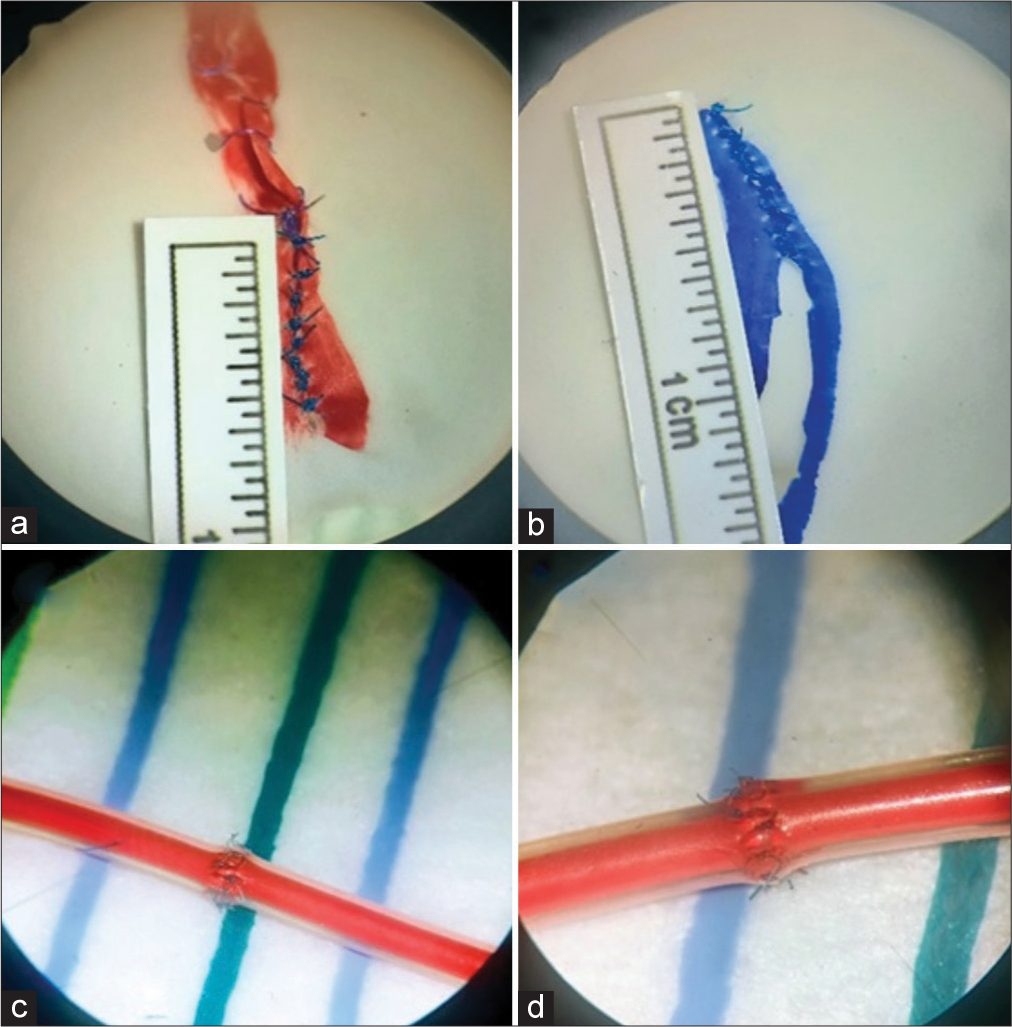
These practices were divided into three subgroups: (a) microsuture, (b) fine movements, and (c) microanastomosis. Each of these subgroups, in turn, consists of two practices. For microsuture practices, we used latex gloves stretched on wooden plates cut with a central hole or tensioned in large plastic lids, on which the 10 mm “incision” was marked [ Microsutures 1 (With latex glove stretched on a wooden plate or lid) Fine movements 1 (With cigarette and tobacco leaves) Fine movements 2 (With gauze) Microsutures 2 (We repeat the practice of microsutures and compare the improvement in quality and time) Microanastomosis 1 (term-terminal in silastic tube) Microanastomosis 2 (laterolateral in silastic tube, longitudinal repair).
For the practices of microanastomosis, silastic tubes were used (caliber/diameter of the neonatal proximal catheter of ventriculoperitoneal shunt) fixed with adhesive cloth to the working tray and cross-sections were made for termino terminal anastomosis, and longitudinal cuts of 10 mm for longitudinal repair,[
Practices in biological specimens (placental vessels)
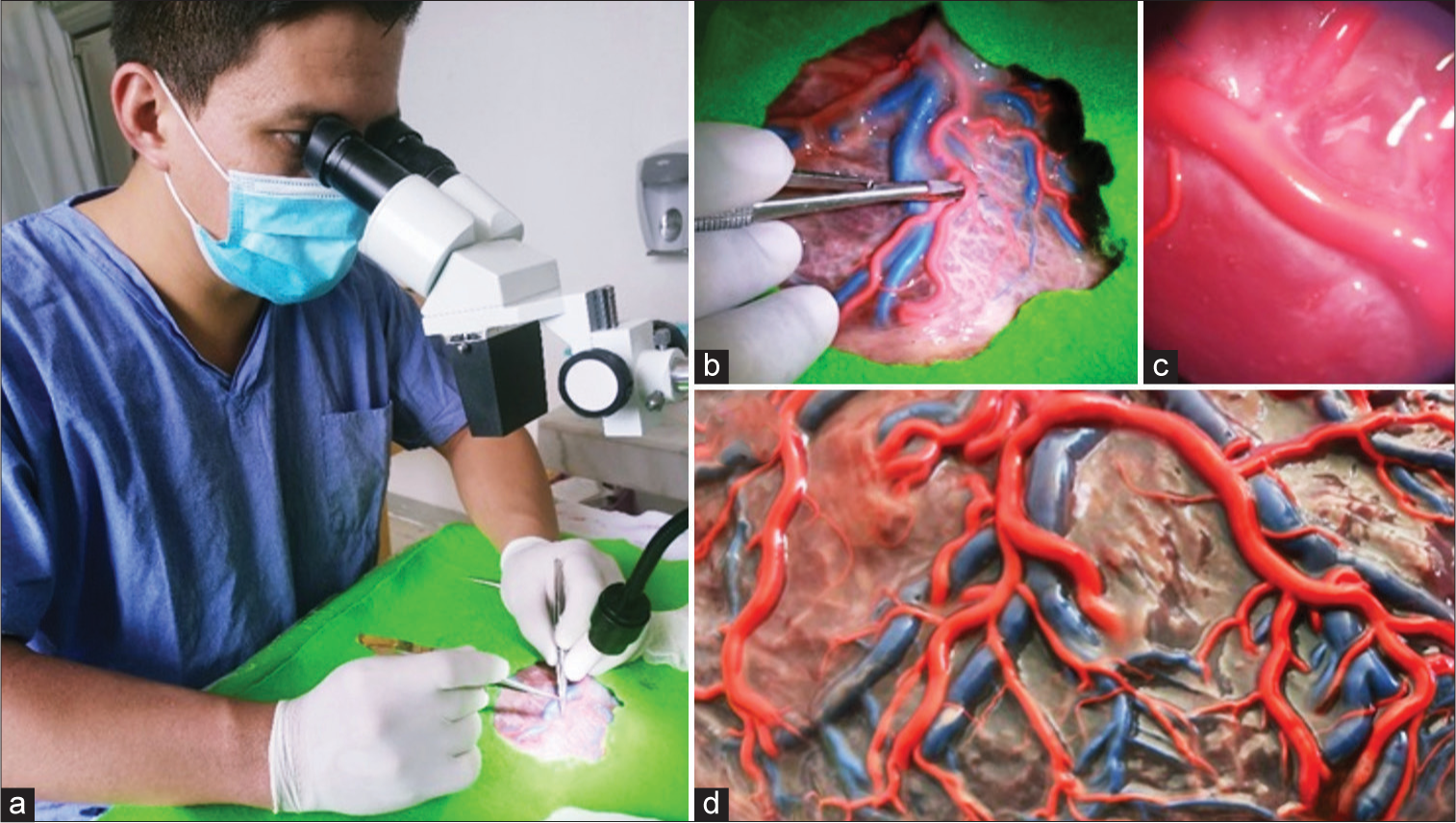
After the acquisition of placental specimens, we used the technique of repletion with liquid latex and acrylic paint, with red color for the arteries, and blue color for the veins.[ Placental vessel filling technique Microdissection of placental vessels Simulation of microdissection according to the disposition of cerebral vessels Sylvan Valley microdissection simulation; in addition, it is possible to simulate clipping aneurysms with Fogarty catheters and tumor resection with colored silicone.[
Practices in brain specimens obtained after the conclusion of the autopsy
In cases of autopsy, after the adequate extraction and detailed macroscopic analysis were finished by the forensic department, rigorous selection criteria were established in accordance with our previously mentioned agreement with this department; furthermore, each specimen (two brains) was fixed.
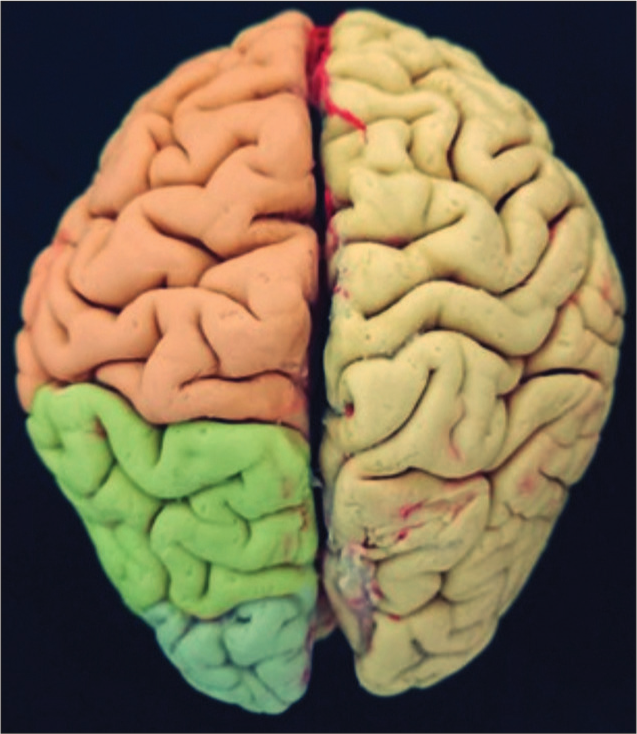
Descriptive anatomical photographs of the cerebral vasculature were obtained, including cerebral vasculature, arachnoid, and vessel microdissection exposed the anatomy of the cerebral cortex, also the lateral and basal faces [
Cerebral vessel filling technique;[ Macroscopic Study and Microdissection of Grooves, Gyres, and Cerebral Vessels Ventricular puncture workshop Approaches (e.g., amygdalohippocampectomy) without damaging the anatomical structures of interest for the inclusion of the autopsy lamellae Klingler method and white fiber dissection.
Figure 4:
(a) Model of a skull printed in 3D dental resin. (b) Placental specimens are easily accessible in any hospital that has an established gynecology and obstetrics service and can be obtained through local agreements with such a service whenever necessary. (c) Cadaveric specimen donated by the institute of pathological anatomy. (d) Cadaveric specimen in which latex and dye were injected into the blood vessels.
Practices in 3d printing models
We developed a self-made 3D skull model using volunteers for 3D skull modeling. This method offers a convenient and cost-effective alternative for 3D-printed skulls; these models allow the execution of different approaches during drilling workshops. Through our experience, we have found that dental resin is the material that more closely resembles bone in terms of its properties and characteristics.[
Basic microsurgery laboratory in a second-level hospital
The dissection stations should mimic the operating room environment.[
Microsuture, fine movements microanastomosis
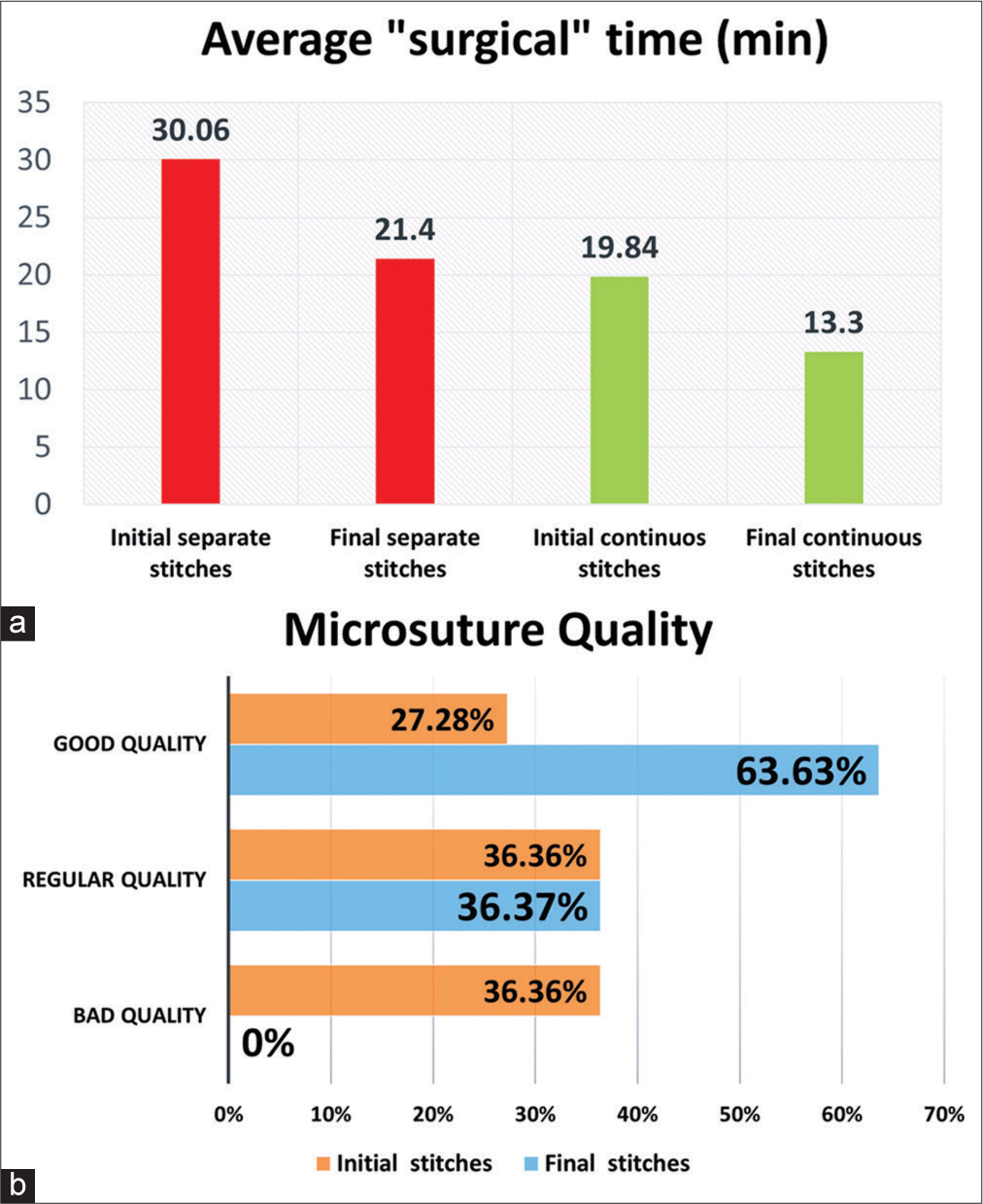
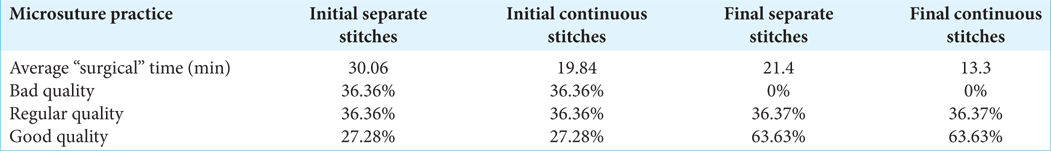
Time and quality of skills were made in 3 weeks. After timing 11 residents when performing microsutures for the 1st time of ten separate simple stitches and ten continuous single stitches, the average times for each type of suture obtained were 30.06 min and 19.84 min, respectively; in addition, we analyzed the quality, firmness, and resistance of the stitches, the distance between stitches and adequate knot, classifying them in three categories, as good quality 36.36%, fair quality 36.36% if features one failed feature, and poor quality 27.27% if two or more of the features failed.
After this, the practices of fine movements were performed. Then, the time and quality of the microsutures were timed again, obtaining a notable decrease in the “surgical time,” is the new averages of 21.4 min and 13.33 min, for the separate single interrupted and continuous single stitches, respectively, after three weeks the average decrease to 8.65 min for the separated single stitches and 6.51 min for the continuous single stitches. There was also an increase in the quality of the stitches and the suture pattern, reducing the space between each stitch and finding more resistant knots; good quality 63.63% and regular quality 36.37% improved significantly after the aforementioned weeks. In addition, there was a marked improvement in the technique and handling of the microsurgical instruments, with many of the residents describing greater self-confidence [
Figure 6:
(a) It shows the average “surgical” time. A decrease in the average “surgical” microsuture was obtained, with an average decrease of 8.65 min for the separated single stitches and 6.51 min for the continuous single stitches. (b) It shows the improvement in the quality of the stitches, according to the mentioned parameters, after the microsuture and fine movement practices.
Table 1:
Initial and final results after performing the practices of microsuture and fine movements. A decrease in the average “surgical microsuture” was obtained, as well as an increase in the quality of the stitches improving the suture pattern, reducing the space between each stitch and finding more resistant stitches.
Placental vessels
A satisfactory and high-quality replenishment was obtained in all the specimens used after a brief explanation and support of video material on the technique, as well as advice by telephone from a vascular and skull base neurosurgeon with experience in the field. In addition, an adequate simulation was obtained for the practice of microdissection in a small and deep space by placing specimens inside 3D printing skull models [
Descriptive anatomical photographs of the cerebral vasculature and white fibers
Multiple anatomical-descriptive photographs have been obtained from this work, which can be used as an important source of reference and anatomical study and laboratory library.
Figure 9:
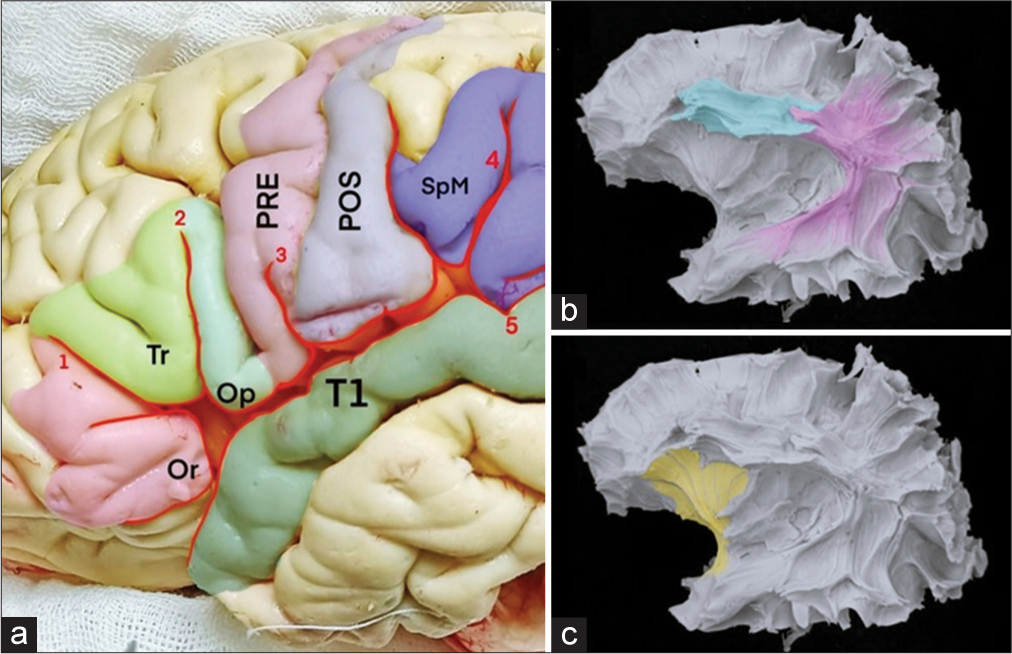
(a) Specimen of the cerebral hemisphere in which the lateral sulcus can be observed. (b and c) With the Klinger technique, the arcuate fasciculus can be observed in one of the cerebral specimens. Or: pars orbitalis; Tr: pars triangularis; Op: pars opercularis; PRE: precentral gyrus; POS: postcentral gyrus; SpM: supramarginal gyrus; T1: superior temporal gyrus.
DISCUSSION
Establishing an affordable microsurgery laboratory in low-income countries presents numerous challenges due to limited resources and financial constraints. Hosain[
Another important challenge in establishing microsurgery laboratories is the limited availability and high-cost equipment and resources. Hopefully, we addressed enough of this challenge by providing recommendations for affordable alternatives, such as refurbished or donated instruments, collaborating with medical device manufacturers for discounted rates, and exploring local suppliers. By leveraging these cost-effective options, low-income hospitals can overcome financial constraints and acquire the necessary equipment for their microsurgery laboratory. Another important aspect discussed in this guide is the setup and optimization of the microsurgery laboratory. Recommendations are provided for the layout, ergonomic considerations, lighting, and sterilization processes to ensure a safe and efficient learning environment. These suggestions must consider the potential problem of limited space and aim to maximize the functionality of the laboratory while keeping costs low. The development of training protocols and a tailored curriculum is crucial for enhancing microsurgical skills in neurosurgery residents.[
This guide emphasizes the importance of comprehensive training, including skill-building exercises, progressive learning modules, and hands-on training techniques that can be implemented within the laboratory. In addition, the integration of simulation-based training and virtual reality tools is explored as a cost-effective option to augment the microsurgical training experience. By incorporating these innovative training methods, residents can further enhance their skills in a controlled and immersive environment. Quality control plays a significant role in maintaining the effectiveness of the microsurgery laboratory. This guide highlights the importance of regular equipment maintenance, instrument sterilization, and protocols for infection control. By establishing and following these guidelines, hospitals can ensure a safe and hygienic learning environment for residents, minimizing the risk of personnel contamination and keeping patients safe.
Furthermore, this guide emphasizes the value of knowledge sharing and collaboration. It provides strategies for fostering partnerships with external organizations or institutions that can provide support, resources, or mentorship. The increasing use of virtual reality research, virtual meetings, online education initiatives, and telehealth, initially introduced out of necessity during the COVID-19 pandemic, represent interesting tools to expand their knowledge and research among low-income country residents.[
The impact and effectiveness of the established microsurgery laboratory can be evaluated through a well-designed evaluation framework. This guide suggests gathering feedback, tracking progress, and measuring outcomes to improve the training program continuously. By assessing the impact of the laboratory on improving microsurgical skills and reducing morbidity rates and complications associated with neurological surgeries, hospitals can further refine their training protocols and curricula. We have a consensus on what was mentioned by Joseph et al.,[
CONCLUSION
Establishing an affordable microsurgery laboratory in low-income countries is a challenging but achievable goal. This guide has outlined what we believe to be some key steps and objectives involved in setting up a low-cost microsurgery laboratory tailored to the specific needs of neurosurgery residents. We emphasize the importance of identifying cost-effective alternatives for equipment sourcing, such as refurbished or donated instruments, collaborating with manufacturers for discounted rates, and exploring local suppliers. These strategies can help overcome financial barriers and ensure the availability of necessary equipment. Hopefully, this comprehensive guide could serve as a roadmap for establishing an affordable microsurgery laboratory in low-income countries. By addressing the challenges, providing recommendations, and offering strategies, this guide aims to empower neurosurgery residents and show these countries that by enhancing the microsurgical skills of trainees, there is better patient care, fewer complications, and shorter hospital stays.
Ethical approval
This study was approved by the Bioethics Committee of the RUDN University, Russian Academy of Sciences named after B.V. Petrovsky, Moscow, Russian Federation (Jan 10th, 2022).
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abraham I, Lewandrowski KU, Elfar JC, Li ZM, Fiorelli RK, Pereira MG. Randomized clinical trials and observational tribulations: Providing clinical evidence for personalized surgical pain management care models. J Pers Med. 2023. 13: 1044
2. Altunrende ME, Hamamcioglu MK, Hıcdonmez T, Akcakaya MO, Bırgılı B, Cobanoglu S. Microsurgical training model for residents to approach to the orbit and the optic nerve in fresh cadaveric sheep cranium. J Neurosci Rural Pract. 2014. 5: 151-4
3. Attebery JE, Nuwas E, Mayegga E, Rabiel H, Massaga FA, Elahi C. Global neurosurgery: A retrospective cohort study to compare the effectiveness of two training methods in resource-poor settings. Neurosurgery. 2023. 4: 1
4. Baldoncini M, Campero A, Pérez Cruz JC, Recalde R, Parraga R, Sanchez Gonzalez FJ. Microsurgical anatomy and approaches to the cerebral central core. World Neurosurg. 2019. 129: e23-34
5. Camacho García FJ, Ramírez León JF, Rojas Galvis MA, Cortés Barré M, Cogua Cogua LN. Microsurgery guide on training techniques in minimally invasive surgery. Rev Colombi Ortop Traumatol. 2019. 33: 84-90
6. Cherian I, Yi G, Munakomi S. Cisternostomy: Replacing the age old decompressive hemicraniectomy?. Asian J Neurosurg. 2013. 8: 132-8
7. Chung SB, Ryu J, Chung Y, Lee SH, Choi SK. An affordable microsurgical training system for a beginning neurosurgeon: How to realize the self-training laboratory. World Neurosurg. 2017. 105: 369-74
8. Dave A, Singhal M, Tiwari R, Chauhan S, De M. Effectiveness of a microsurgery training program using a chicken wing model. J Plast Surg Hand Surg. 2022. 56: 191-7
9. De Jesus Encarnacion Ramirez M, Peralta I, Ramirez I, Dauly V, Mainer G, Nurmukhametov R. Development of a novel low-cost exoscope to expand access to microneurosurgical care in low-and middle-income countries. World Neurosurg. 2022. 163: 5-10
10. Encarnacion M, Nurmukhametov R, Barrientos RE, Melchenko D, Goncharov E, Bernard E. Anatomical variations of the median nerve: A cadaveric study. Neurol Int. 2022. 14: 664-72
11. Encarnacion Ramirez M, Peralta Baez I, Nurmukhametov R, Goncharov E, Efe IE, Sufianov A. Comparative survey study of the use of a low cost exoscope vs. microscope for anterior cervical discectomy and fusion (ACDF). Front Med Technol. 2022. 4: 1055189
12. Encarnacion Ramirez M, Ramirez Pena I, Barrientos Castillo RE, Sufianov A, Goncharov E, Soriano Sanchez JA. Development of a 3D printed brain model with vasculature for neurosurgical procedure visualisation and training. Biomedicines. 2023. 11: 330
13. Encarnacion Ramirez MD, Musa G, Barrientos Castillo RE, Cherian I, Sufianov A, Lafuente J. Three-dimensional cerebrovascular bypass training. A new low-cost home-made model. Front Med Case Rep. 2021. 2: 1-10
14. Fortunato GM, Sigismondi S, Nicoletta M, Condino S, Montemurro N, Vozzi G. Analysis of the robotic-based in situ bioprinting workflow for the regeneration of damaged tissues through a case study. Bioengineering (Basel). 2023. 10: 560
15. Gómez-Vega JC, Mancera Pérez J, Reghin Neto M, Holanda V, de Oliveira E. Development of microsurgical techniques using vascular staining with and without silicone in human placenta with 3D cranial models. Rev Argent Neuroc. 2021. 35: 160-71
16. Hosain MM. Microsurgery training: Are the live models era coming in to the tail?-a literature review. J Adv Med Med Res. 2021. 33: 97-109
17. Iyer S, Bovonratwet P, Samartzis D, Schoenfeld AJ, An HS, Awwad W. Appropriate telemedicine utilization in spine surgery: Results from a Delphi study. Spine (Phila Pa 1976). 2022. 47: 583-90
18. Javid P, Aydın A, Mohanna PN, Dasgupta P, Ahmed K. Current status of simulation and training models in microsurgery: A systematic review. Microsurgery. 2019. 39: 655-68
19. Joseph FJ, Vanluchene HE, Bervini D. Simulation training approaches in intracranial aneurysm surgery-a systematic review. Neurosurg Rev. 2023. 46: 101
20. Juratli MA, Becker F, Palmes D, Stöppeler S, Bahde R, Kebschull L. Microsurgical training course for clinicians and scientists: A 10-year experience at the Münster university hospital. BMC Med Educ. 2021. 21: 295
21. Lewandrowski KU, Elfar JC, Li ZM, Burkhardt BW, Lorio MP, Winkler PA. The changing environment in postgraduate education in orthopedic surgery and neurosurgery and its impact on technology-driven targeted interventional and surgical pain management: Perspectives from Europe, Latin America, Asia, and the United States. J Pers Med. 2023. 13: 852
22. Liu JK, Kshettry VR, Recinos PF, Kamian K, Schlenk RP, Benzel EC. Establishing a surgical skills laboratory and dissection curriculum for neurosurgical residency training. J Neurosurg. 2015. 123: 1331-8
23. Lizana J, Montemurro N, Aliaga N, Marani W, Tanikawa R. From textbook to patient: A practical guide to train the end-to-side microvascular anastomosis. Br J Neurosurg. 2023. 37: 116-20
24. Malhotra K, Wong BN, Lee S, Franco H, Singh C, Cabrera Silva LA. Role of artificial intelligence in global surgery: A review of opportunities and challenges. Cureus. 2023. 15: e43192
25. Marian-Magaña R, Sangrador-Deitos MV, GuintoNishimura GY, Ballesteros-Herrera D, Cano-Velazquez G, Canela-Calderón OJ. Microsurgical training through laboratory experience: A step-by-step practical guideline. Interdicip Neurosurg. 2022. 7: 101400
26. Martins PN, Montero EF. Basic microsurgery training: Comments and proposal. Acta Cir Bras. 2007. 22: 79-81
27. Matsushima T, Matsushima K, Kobayashi S, Lister JR, Morcos JJ. The microneurosurgical anatomy legacy of Albert L. Rhoton Jr MD: An analysis of transition and evolution over 50 years. J Neurosurg. 2018. 129: 1331-41
28. Montemurro N, Aliaga N, Graff P, Escribano A, Lizana J. New targets and new technologies in the treatment of Parkinson’s disease: A narrative review. Int J Environ Res Public Health. 2022. 19: 8799
29. Montemurro N, Condino S, Carbone M, Cattari N, D’Amato R, Cutolo F. Brain tumor and augmented reality: New technologies for the future. Int J Environ Res Public Health. 2022. 19: 6347
30. Montemurro N, Scerrati A, Ricciardi L, Trevisi G. The exoscope in neurosurgery: An overview of the current literature of intraoperative use in brain and spine surgery. J Clin Med. 2021. 11: 223
31. Montemurro N. Telemedicine: Could it represent a new problem for spine surgeons to solve?. Global Spine J. 2022. 12: 1306-7
32. Muñoz Cobos A, Lecona Butrón H, editors. Manual práctico de microcirugía cirugía vascular y nerviosa. Mexico: Tercer Escalón Editoriales; 2017. 1: 15
33. Ocampo-Navia MI, Gómez-Vega JC. Techniques for the anatomical study of the brain: A review. Univ Med. 2021. 62: 22-32
34. Pahwa B, Singh N, Singh G, Chavda V, Montemurro N, Chaurasia B. Surgical approaches to cavernous sinus: A narrative review of the literature with anatomical drawings. J Neurol Surg A Cent Eur Neurosurg. 2022. 30: 1
35. Pérez-Zabala J, Beldi F. Comprehensive microsurgical and neuroendovascular training model with human placenta. Rev Argent Neuroc. 2020. 34: 300-14
36. Ramirez ME, Sanchez JS, Barsa JL, Barrietos R, Musa G. Training in cerebral aneurysm clipping using self-made 3-dimensional models including blood flow. Brain Spine. 2021. 1: 100815
37. Ramirez MJ, Nurmukhametov R, Musa G, Barrientos Castillo RE, Encarnacion VL, Soriano Sanchez JA. Three-dimensional plastic modeling on bone frames for cost-effective neuroanatomy teaching. Cureus. 2022. 14: e27472
38. Reynoso JP, De Jesus Encarnacion M, Nurmukhametov R, Melchenko D, Efe IE, Goncharov E. Anatomical variations of the sciatic nerve exit from the pelvis and its relationship with the piriformis muscle: A cadaveric study. Neurol Int. 2022. 14: 894-902
39. Samprónf N. Manual de microcirugia neurovascular. Donostia España: Comunicación OSI Donostialdea; 2017. 1: 10
40. Siragusa L, Angelico R, Angrisani M, Zampogna B, Materazzo M, Sorge R. How future surgery will benefit from SARS-COV-2-related measures: A SPIGC survey conveying the perspective of Italian surgeons. Updates Surg. 2023. 75: 1711-27
41. Uhl JF, Sufianov A, Ruiz C, Iakimov Y, Mogorron HJ, Encarnacion Ramirez M. The use of 3D printed models for surgical simulation of cranioplasty in craniosynostosis as training and education. Brain Sci. 2023. 13: 894
42. Wen HT, Matsushima T, Morcos JJ. Rhoton Society, Evandro Pinto da Luz de Oliveira, MD, PhD (November 8, 1945-February 11, 2021): A giant of microneurosurgery and neuroanatomy. Neurosurgery. 2021. 89: E229-32
43. Yadav YR, Parihar V, Ratre S, Kher Y, Iqbal M. Microneurosurgical skills training. J Neurol Surg A Cent Eur Neurosurg. 2016. 77: 146-54
44. Yaşargil MG. A legacy of microneurosurgery: Memoirs, lessons, and axioms. Neurosurgery. 1999. 45: 1025-92






Alan Lira
Posted January 2, 2024, 6:00 am
Exelent job!!! Goooo my lord salvador!!!!!!
Maria Teresa Flores Quezada
Posted January 2, 2024, 6:11 am
Un artículo muy completo, con información de gran ayuda para la orientación de los laboratorios de microcirugía en formación.
José Luis García Munguía
Posted January 2, 2024, 6:53 am
Excelente artículo, muchas felicidades! Muy interesante y cierto
Martin Mendez Guerra
Posted January 2, 2024, 8:16 am
The best practices and trained personnel begin with a well-structured academic environment, although, sometimes, this is a problematic approach in limited-resource countries; this is where this excellent paper makes a difference. In conclusion, we all want the best healthcare for our loved ones; this paper begins the journey.
Emmanuel Contreras
Posted January 2, 2024, 4:37 pm
An excellent review and research article. Quality of knowledge is the main thing and the key to teaching health sciences, the limitation of resources for the transmission of this knowledge generates an area of opportunity to be corrected in the training of professionals.