- Department of Surgery, Section of Neurosurgery, The Aga Khan University Hospital, Karachi, Pakistan
Correspondence Address:
Syed Ather Enam
Department of Surgery, Section of Neurosurgery, The Aga Khan University Hospital, Karachi, Pakistan
DOI:10.4103/sni.sni_77_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Muhammad Waqas, Muzna Iftikhar, Usman T. Siddiqui, Syed Ather Enam. Ependymal enhancement on magnetic resonance imaging for the identification of high-grade gliomas. 26-Sep-2017;8:227
How to cite this URL: Muhammad Waqas, Muzna Iftikhar, Usman T. Siddiqui, Syed Ather Enam. Ependymal enhancement on magnetic resonance imaging for the identification of high-grade gliomas. 26-Sep-2017;8:227. Available from: http://surgicalneurologyint.com/surgicalint-articles/ependymal-enhancement-on-magnetic-resonance-imaging-for-the-identification-of-high%e2%80%91grade-gliomas/
Abstract
Background:High-grade gliomas have high infiltrative potential and spread along white matter and blood vessels. Enhancement of ependymal lining on magnetic resonance imaging (MRI) is considered as a marker of parenchymal spread of disease. In this study, we aimed to assess the sensitivity, specificity, and positive and negative predictive values of ependymal enhancement (EE) for identification of high-grade glial tumors.
Methods:We reviewed preoperative MRI scans of 94 consecutive patients surgically treated for space occupying lesions of the brain for EE. Assessment for EE was blind to the final histopathological diagnosis of the patient. An enhancement of more than 2 mm was considered positive. Pathologies of these patients were reviewed and matched to the radiological findings. Percentage and proportion of EE in glial and non-glial pathology groups was then calculated and a sensitivity and specificity analysis was performed.
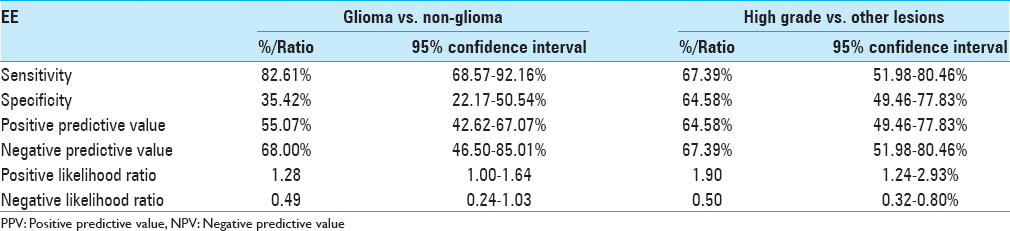
Results:The population included 94 cases (64 males and 30 females) with population mean age 45 ± 15.5 years. Sensitivity of EE in differentiating glioma from total number of cases was 82.61% specificity 35.42% (P value = 0.048). EE had a sensitivity of 67.39% and specificity of 64.58% (P value = 0.002) in identifying high-grade glioma within the glioma group with a positive predictive value of 64.58% (95% CI: 49.46% to 77.83%), negative predictive value of 67.39% (95% CI: 51.98% to 80.46%).
Conclusion:EE has moderate sensitivity and specificity for high-grade gliomas. However, larger sample studies are required for further validation of this observations.
Keywords: Ependymal enhancement, high-grade glioma, tumor spread
INTRODUCTION
Gliomas are the most common primary neoplasms of the brain. These include astrocytomas, oligodendroglioma, and oligoastrocytomas, based on the originating cell. Astrocytomas and oligodendrogliomas are further subdivided based on the histological grade. High-grade gliomas include anaplastic astrocytoma (Grade III astrocytoma), anaplastic oligodendroglioma (Grade III aoligodendroglioma), and glioblastoma multiforme (GBM, Grade IV astrocytoma).[
High-grade glioma cells invade beyond the grossly appreciable tumor margins. This migration of cells is along white matter and blood vessels,[
Imaging characteristics such as degree of contrast area, tumor necrosis volume, and edema surrounding tumor have been well-identified as prognostic markers in high-grade lesion.[
MATERIALS AND METHODS
This was a retrospective diagnostic study conducted at our institution from 1st January 2013 to 31st December 2015. The review period was from 1st March 2016 to 31st May 2016. The Aga Khan University.
Participants
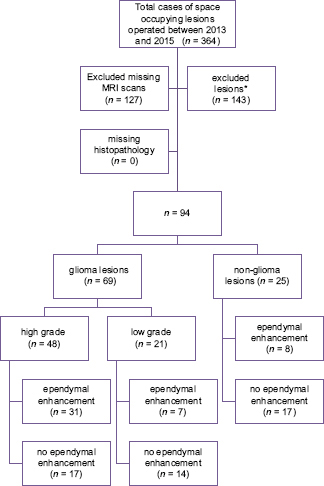
We included consecutive patients undergoing surgery for space occupying lesions of the brain with preoperative magnetic resonance imaging (MRI) brain available in hospital records for the review and definitive histopathology reports. Patients with inconclusive histopathology reports and missing preoperative MRI scans were excluded from the study. Patient selection is shown in
Test method
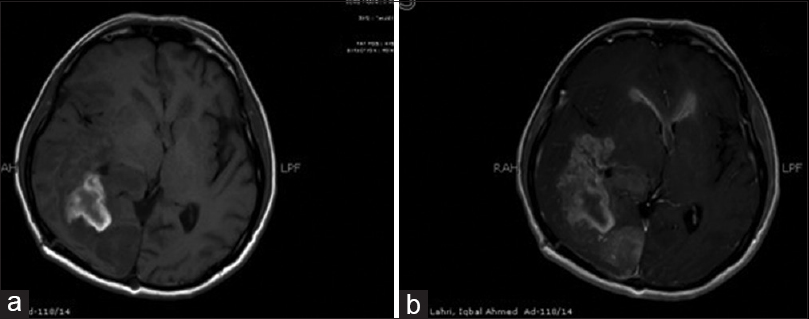
Preoperative 1.5T MRI scans were reviewed for EE. T1-weighted (T1W) non-enhanced axial images were compared to corresponding T1W contrast enhanced axial images on picture archiving and communication system (PACS). Enhancement of the ependymal lining more than 2 mm was considered positive. An example of this method is given in
Statistical analysis
Data was analyzed using IBM SPSS Statistics Version 20 (Chicago, Illinois). Descriptive analysis was done for demographic data. Frequencies and proportions of different pathologies and radiological characteristics were calculated. The sensitivity, specificity, positive, and negative predictive value were calculated by using a 2 × 2 contingency table. Statistical analysis was done using Pearson Chi-square test. P value was calculated and P < 0.05 was considered significant. Univariate and multivariate logistic regression analysis was applied including variables of age, gender, and EE. Cohen kappa (Cohen K) value was calculated to assess the inter-rater agreement.
RESULTS
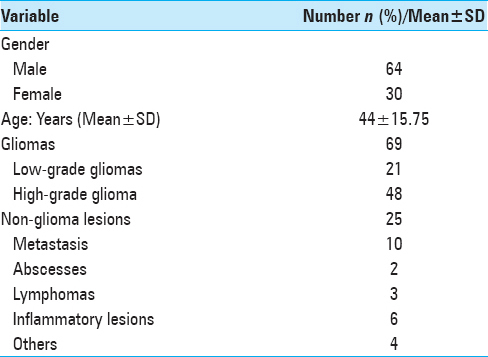
Ninety-four patients were included in the study. Of a total of 94 cases, 64 were males and 30 females with a mean age of 44.4 ± 15.75. The procedures included 13 neuronavigation-guided biopsies, 58 craniotomies with excision of lesion, and 23 neuronavigation guided biopsy with excision.
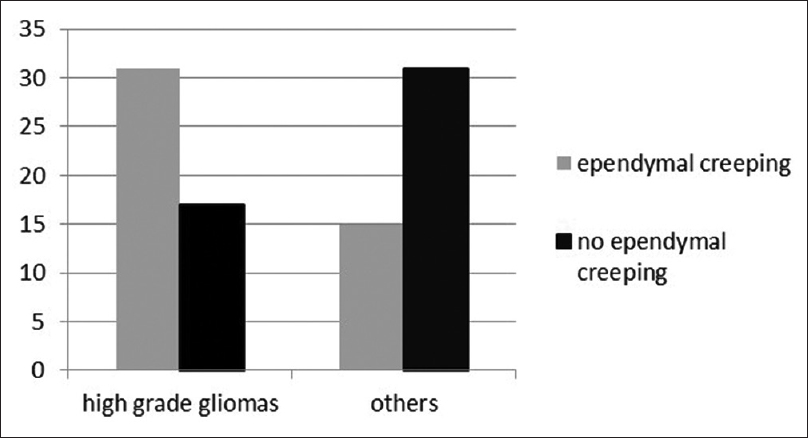
69 gliomas and 25 non-glial lesions were identified [
Glial vs. non-glial lesions
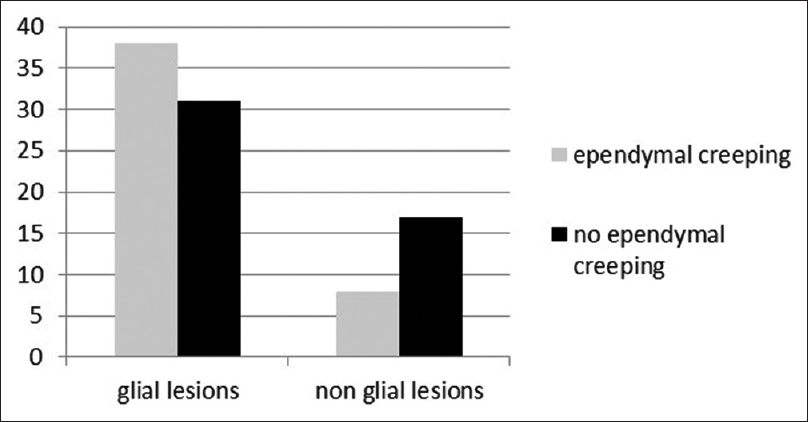
EE was identified in 38 (55.07%) cases in glial cases and 8 (32%) in non-glioma cases (P value 0.048) [
The positive predictive value was 55.07% (95% CI: 42.62% to 67.07%) and a negative predictive value of 68.00% (95% CI: 46.50% to 85.01%). Positive likelihood ratio was 1.28 (95% CI: 1.00 to 1.64) and the negative likelihood ratio was 0.49 (95% CI: 0.24 to 1.03). Diagnostic odds ratio (OR) = 2.61.
High-grade glial vs. all other lesions
Out of the total 48 high-grade lesion 64.58% showed EE, compared with 32.60% in other lesions, P value of 0.002 [
According to univariate analysis OR for EE as a predictor of high-grade glioma was 0.30.6 (95% CI = 0.134 to 0.702, P value 0.005). On multivariate regression analysis including variables of age and gender, EE was an independent predictor of a high-grade pathology with an OR of 0.34 (95% CI = 0.144 to 0.810, P value = 0.05). OR of EE as a predictor of glial neoplasm was however, not statistically significant with OR = 0. 559 (CI = 0.21 to 1.43, P value = 0.228). Cohen k value for inter rater agreement was 0.89 with standard error of 0.45.
DISCUSSION
The usual presentation of gliomas on MRI scans include hypo or iso-intensity on T1W imaging and hyperintensity on T2 imaging.[
Subependymal region has long been considered a common site for tumor invasion. It is one of the several structures in the brain called Scherer's secondary structures where glioma cells tend to migrate.[
EE has also been investigated with multiple pathologies such as viral etiology, inflammatory lesions, meningeal carcinomatosis, meningitis, and ventriculitis and very rarely Whipple's disease or sarcoidosis. Nodular periventricular enhancement may be present in central nervous system (CNS) lymphomas or other neoplastic processes.[
In the light of existing evidence, we decided to assess the value EE on preoperative imaging in determining final pathology. In our experience, EE has a sensitivity of 82.61% and negative predictive value 68% in glioma vs. non-glioma lesions and its absence may be a useful tool in ruling out the possibility of a lesion being a glioma. It is also important to note that none of our patients with infectious pathology showed EE on MRI. In countries, where tuberculosis is endemic many neurologists and neurosurgeons treat patients with enhancing lesions using anti-tuberculosis treatment without cultures or tissue diagnosis.
The Cohen K value was 0.89 which according to interpretation of Cohen K provided by Landis et al. indicates excellent agreement.[
We found that the frequency of EE is significantly higher in high-grade gliomas yet it is not an exclusive attribute of higher grade. Number of cases with EE in non-glial lesion and low-grade glioma group though smaller (32% and 33.33% respectively), is not negligible. Therefore, feature of EE fails to achieve near 100% sensitivity and specificity.
To best of our knowledge this is the first study that specifically tries to evaluate EE in distinguishing high-grade glial neoplasms. Preoperative MRI scans and histopathology reports were reviewed independently. To minimize bias reviewers were kept blind to the histopathology reports and features of MRI images. All MRI scans were obtained from one institution and the MRI protocols were uniform and conducted on the same MRI machine.
The study has several limitations. It has a retrospective design. The sample size was limited as a significant number of cases could not be included in the final analysis because of unavailability of preoperative scans at the time of review, primarily due to scans being generated outside the institution. Different patients presented at different times from the onset of symptoms there were at different stage of disease which could affect the presence or absence of EE. A follow-up MRI and overall survival, where possible, could answer the question of ependymal involvement with disease progression. Further studies need to be done regarding use of EE to assess grade, disease prognosis, multicentricity, recurrence, and detect change in grade on recurrence of a previously low-grade lesion.
CONCLUSION
EE has moderate sensitivity and specificity for high-grade gliomas. However, larger sample studies are required for further validation of these observations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Berger M WC.editorsThe gliomas. Philadelphia: W.B Saunders; 1999. p.
2. Enam SA, Eisenberg AD, Norman D, Rosenblum ML. Patterns of spread and recurrence of glioma: Studies by neuroimaging. Brain Tumor Invasion: Biological, Clinical and Therapeutic Considerations. 1998. p. 133-59
3. Enam SA, Rosenblum ML, Edvardsen K. Role of extracellular matrix in tumor invasion: Migration of glioma cells along fibronectin-positive mesenchymal cell processes. Neurosurgery. 1998. 42: 599-
4. Enam SA, Rosenblum ML, Edvardsen K. Role of extracellular matrix in tumor invasion: Migration of glioma cells along fibronectin-positive mesenchymal cell processes. Neurosurgery. 1998. 42: 599-608
5. Giese A, Laube B, Zapf S, Mangold U, Westphal M. Glioma cell adhesion and migration on human brain sections. Anticancer Res. 1997. 18: 2435-47
6. Guerini H, Helie O, Leveque C, Adem C, Hauret L, Cordoliani YS. Diagnosis of periventricular ependymal enhancement in MRI in adults. J Neuroradiol. 2003. 30: 46-56
7. Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996. 27: 65-73
8. Harpold HL, Alvord EC, Swanson KR. The evolution of mathematical modeling of glioma proliferation and invasion. J Neuropathol Exp Neurol. 2007. 66: 1-9
9. Iliadis G, Kotoula V, Chatzisotiriou A, Televantou D, Eleftheraki AG, Lambaki S. Volumetric and MGMT parameters in glioblastoma patients: Survival analysis. BMC Cancer. 2012. 12: 3-
10. Kaidar-Person O, Eran A, Darawshe F, Tzuk-Shina T. P17.88 The Clinical Significance of Ependymal Enhancement At Presentation In Patients With Malignant Glioma: Single Center Experience. Neuro-oncology. 2014. 16: ii108-
11. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
12. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33: 159-74
13. Liao W, Liu Y, Wang X, Jiang X, Tang B, Fang J. Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol. 2009. 50: 217-25
14. Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
15. Ludemann L, Grieger W, Wurm R, Budzisch M, Hamm B, Zimmer C. Comparison of dynamic contrast-enhanced MRI with WHO tumor grading for gliomas. Eur Radiol. 2001. 11: 1231-41
16. Matthew L, Whitea , Yan Zhanga PK, Rykenc TC. Can Tumor Contrast Enhancement Be Used as a Criterion for Differentiating Tumor Grades of Oligodendrogliomas?. AJNR. 2005. 26: 784-90
17. Mazurowski MA, Zhang J, Peters KB, Hobbs H. Computer-extracted MR imaging features are associated with survival in glioblastoma patients. J Neurooncol. 2014. 120: 483-8
18. Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009. 100: 2235-41
19. Okamoto K, Ito J, Takahashi N, Ishikawa K, Furusawa T, Tokiguchi S. MRI of high-grade astrocytic tumors: Early appearance and evolution. Neuroradiology. 2002. 44: 395-402
20. Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: Rationale and potential role of targeted agents. The oncologist. 2006. 11: 152-64
21. Roberts HC, Roberts TP, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: Correlation with histologic grade. Am J Neuroradiol. 2000. 21: 891-9
22. Scherer H. Structural development in gliomas. Am J Cancer. 1938. 34: 333-51
23. Schoenegger K, Oberndorfer S, Wuschitz B, Struhal W, Hainfellner J, Prayer D. Peritumoral edema on MRI at initial diagnosis: An independent prognostic factor for glioblastoma?. Eur J Neurol. 2009. 16: 874-8
24. Stupp R, Reni M, Gatta G, Mazza E, Vecht C. Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol. 2007. 63: 72-80
25. Woodward D, Cook J, Tracqui P, Cruywagen G, Murray J, Alvord E. A mathematical model of glioma growth: The effect of extent of surgical resection. Cell Proliferation. 1996. 29: 269-88
26. Young GS, Macklin EA, Setayesh K, Lawson JD, Wen PY, Norden AD. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J Neurooncol. 2011. 104: 261-9