- College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Makkah,
- King Abdullah International Medical Research Center, Jeddah,
- Department of Neurosurgery, King Abdulaziz Medical City, National Guard Health Affairs, Jeddah, Makkah, Saudi Arabia.
Correspondence Address:
Ahmed S. Abdulhamid, College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Makkah, Saudi Arabia.
DOI:10.25259/SNI_136_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical termsHow to cite this article: Ahmed S. Abdulhamid1,2, Abdullah A. Ghaddaf1,2, Abdullah F. Bokhari1,2, Yasir A. Alghamdi1,2, Mohammed F. Alhakami1,2, Ahmad Khalid Alaboud1,2, Ahmed Lary3. Equiosmolar hypertonic saline and mannitol for brain relaxation in patients undergoing supratentorial tumor surgery: A systematic review and meta-analysis. 31-Mar-2022;13:120
How to cite this URL: Ahmed S. Abdulhamid1,2, Abdullah A. Ghaddaf1,2, Abdullah F. Bokhari1,2, Yasir A. Alghamdi1,2, Mohammed F. Alhakami1,2, Ahmad Khalid Alaboud1,2, Ahmed Lary3. Equiosmolar hypertonic saline and mannitol for brain relaxation in patients undergoing supratentorial tumor surgery: A systematic review and meta-analysis. 31-Mar-2022;13:120. Available from: https://surgicalneurologyint.com/surgicalint-articles/equiosmolar-hypertonic-saline-and-mannitol-for-brain-relaxation-in-patients-undergoing-supratentorial-tumor-surgery-a-systematic-review-and-meta-analysis/
Abstract
Background: Hypertonic saline (HS) and mannitol are hyperosmolar agents that are usually used to reduce intracranial pressure (ICP) and provide a satisfactory brain relaxation. The aim of the study was to perform a systematic review and meta-analysis to compare the efficacy of HS and mannitol on brain relaxation intraoperatively in patient undergoing craniotomies for supra-tentorial brain tumors.
Methods: A systematic review and meta-analysis of randomized control trials. We included randomized control trials that compared equiosmolar HS and mannitol in supratentorial tumors craniotomies and reported at least one of the following outcomes: degree of brain relaxation, ICP, central venous pressure, mean arterial pressure, perioperative fluid input, urine output, Na+ levels, and K+ levels. We searched Medline, Cochrane Central Register of Controlled Trials, and Embase using MESH terms and keywords. The bibliographic references of included studies and trial registries were also searched.
Results: Seven articles were included. The degree brain of relaxation was comparable across the two groups with slight tendency toward HS (RR = 1.13, 95% CI 0.99–1.29; P = 0.08). Mannitol was associated with significantly higher urine output (standardized mean difference [SMD] = −1.33, 95% CI −1.56–−1.10; P + levels were higher in HS group (SMD = 1.47, 95% CI 0.86–2.09; P
Conclusion: Both HS and mannitol are associated with satisfactory brain relaxation with a non-statistically significant tendency for HS to achieve better relaxation scores with mannitol resulting in higher urine output while HS with higher Na+ levels.
Keywords: Brain relaxation, Hypertonic saline, Mannitol
INTRODUCTION
Osmotherapy is commonly used to achieve satisfactory intracranial pressure (ICP) and achieving brain relaxation.[
Several randomized controlled trials (RCT) have compared equiosmolar HS and mannitol for brain relaxation in patients undergoing craniotomies, yet there were no consistent results between these trials.[
Hence, we aimed to assess the efficacy of equiosmolar HS and mannitol for the degree of brain relaxation and ICP during craniotomies for supratentorial tumors, along with their effect on systematic hemodynamics and electrolytes.
MATERIALS AND METHODS
We conducted this systematic review and meta-analysis according to our pre-specified protocol and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist.[
Search strategy
We searched Medline, Embase, and Cochrane Central Register of Controlled Trials with no restriction on the date and included only studies written in English. We used Medical Subject Headings (MESH) terms and keywords according to each database. Our used MESH terms and keyword were as following: “HS,” “Mannitol,” “brain relaxation,” “craniotomy,” “brain tumor,” and “supratentorial neoplasms.” This search was extended through searching the following trial registries: Clinical Trials Registry, MetaRegister of Controlled Trials, Australian New Zealand Clinical Trials Registry, and UMIN Clinical Trials Registry. The bibliographic references of the included studies were explored to find any relevant studies to our review. The last search was done early - July 2021.
Eligibility and selection criteria
We included only RCTs that have compared the efficacy of 3% or 3.2% HS and 18% or 20% mannitol for brain relaxation in patients who underwent craniotomy for supratentorial tumors only. We excluded any study that has different neurosurgical pathologies, tumors at any location other than supratentorial, non-equiosmolar HS and mannitol, studies with pediatric age group, and the absence of a full text of the article. For a study to be included in our meta-analysis, the study had to have at least one relevant outcome to this systematic review. Our intended outcomes were the degree of brain relaxation (4-point scale surgeon assessment), monitored ICP, mean arterial pressure (MAP), central venous pressure (CVP), peri-operative fluid input, urine output, and electrolytes levels (Na+ and K+).
Two investigators independently and in duplicate reviewed the titles and abstracts for articles that met our pre-specified criteria. Later, the included articles were assessed through their full-text, and if the full-text was eligible, the same two investigators extracted all relevant data in a pre-established data collection sheet. In the case of any disagreement, a third investigator was needed to resolve the disagreement or give the final decision.
Data synthesis and analysis
Degree of brain relaxation was assessed using a 4-point scale (I = perfectly relaxed, II = satisfactorily relaxed, III = firm brain, and VI = bulging brain). We defined I and II as positive events while the rest were considered negative events, and we used the risk ratio (RR) to represent the results. All other outcomes were assessed using the standardized mean difference (SMD). The latest value reported in the intended outcomes that have different time point measures was extracted. For example, if a study has reported serum Na+ levels in 1 h and 2 h, we extracted 2-h Na+ levels. Fluid output was reported as median and range in one study and was converted to mean and standard deviation using the Wan et al. calculator.[
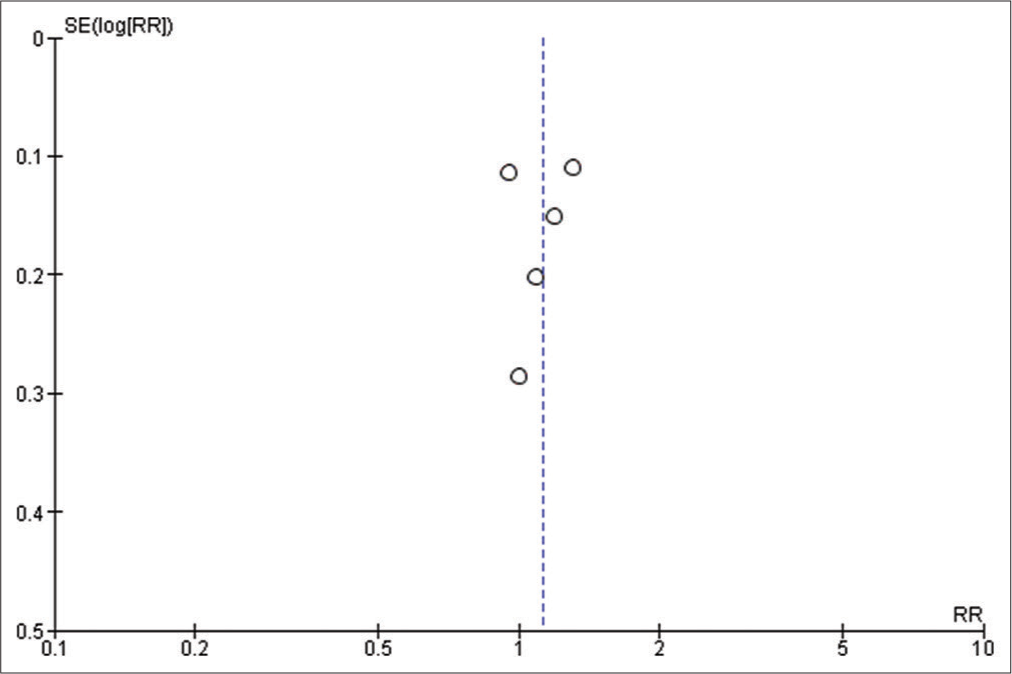
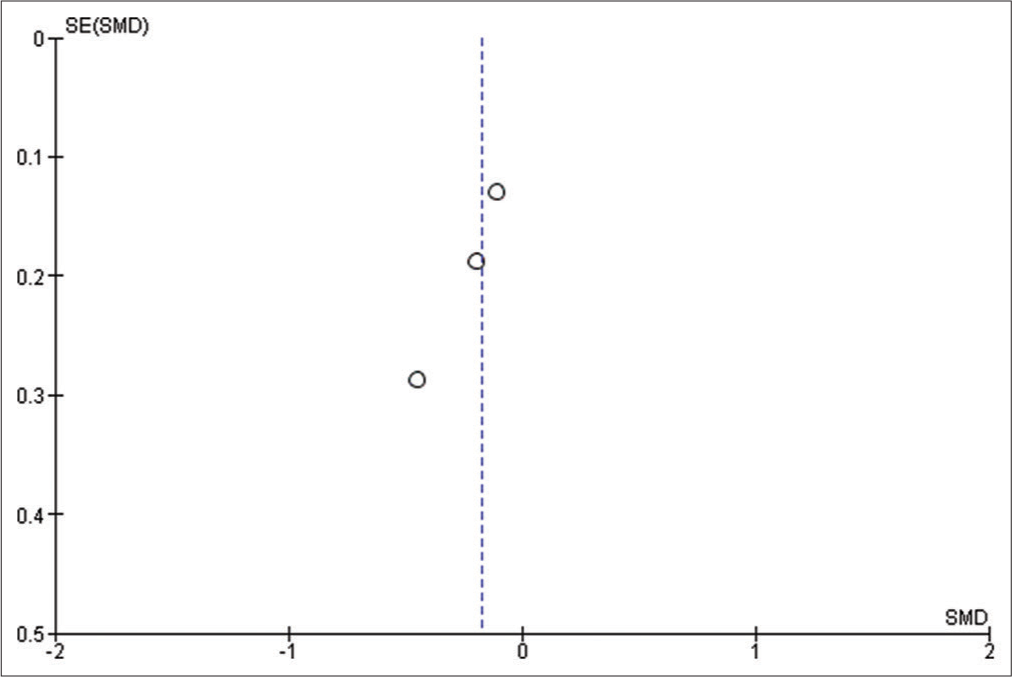
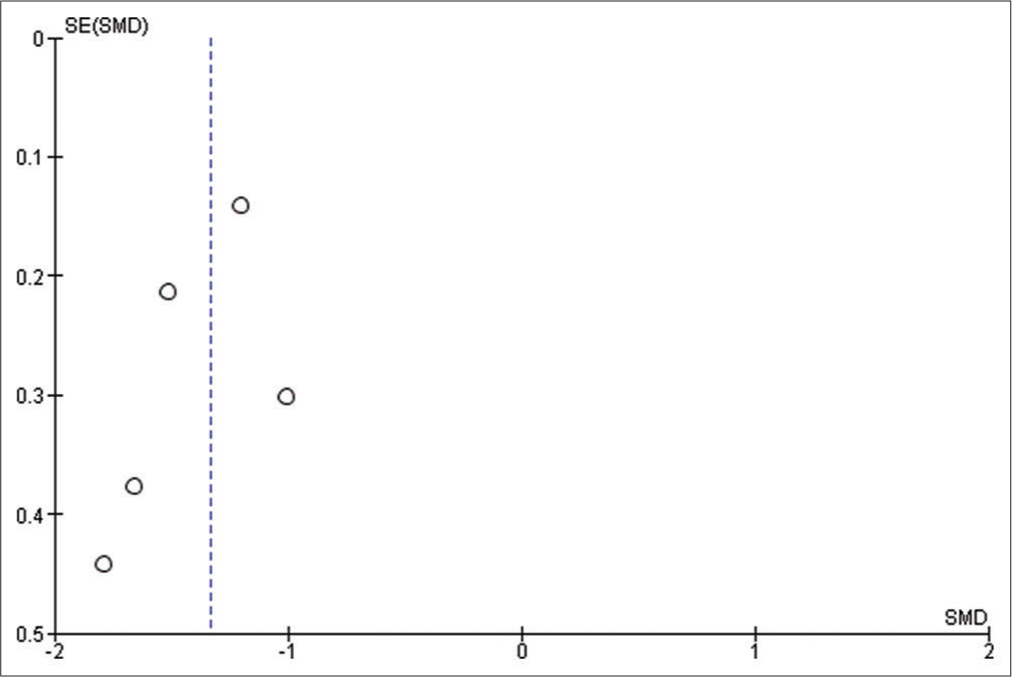
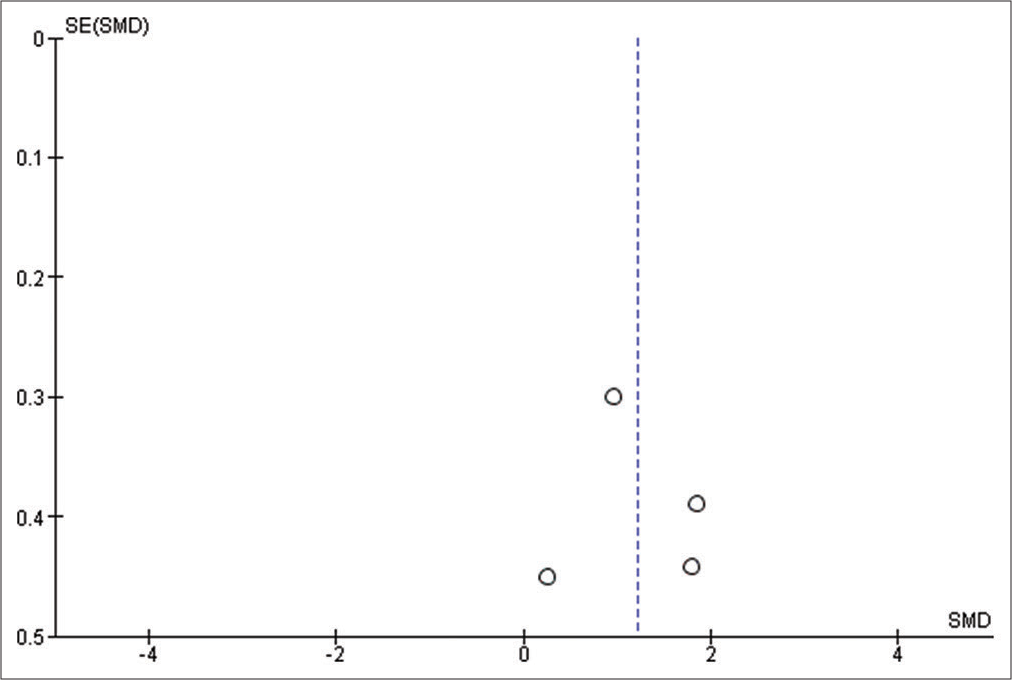
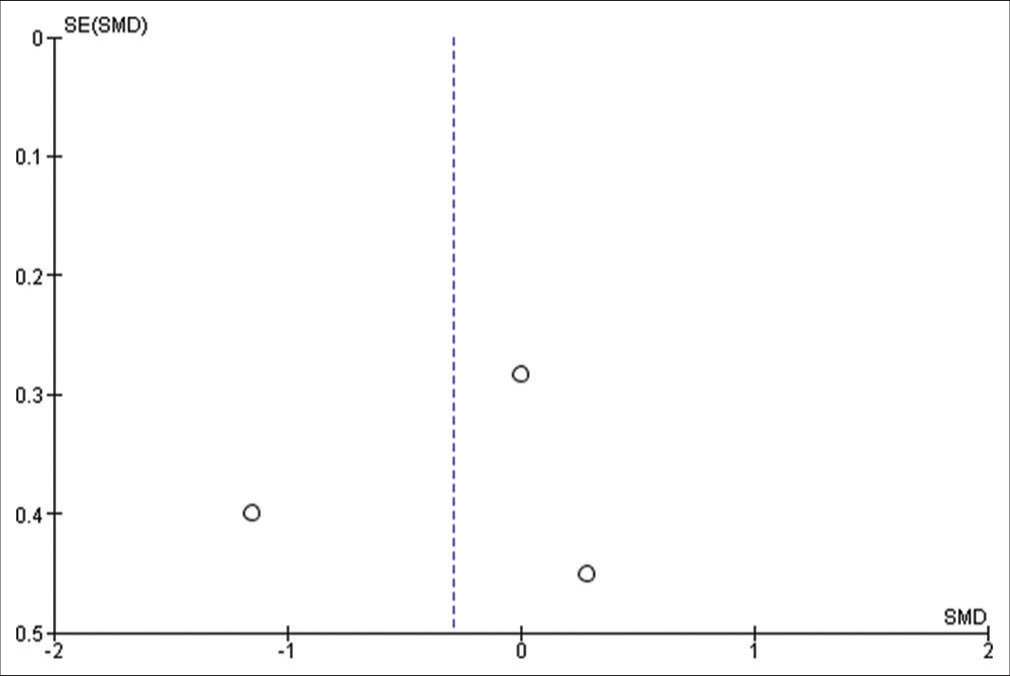
We used Reviewer Manager Software version 5.4 to build our meta-analysis and forest plots. The random-effects model was used for all outcomes. Heterogeneity was assessed using I2 and P-value of Chi-square while visual inspection of the funnel plot was used to assess the publication bias. P < 0.05 was considered as our threshold for statistically significant results; hence, 95% confidence interval was used. Sensitivity analysis was carried out when there is significant heterogeneity, to determine the source of the heterogeneity.
Risk of bias assessment was carried using the Revised Cochrane Risk of Bias Assessment Tool.[
RESULTS
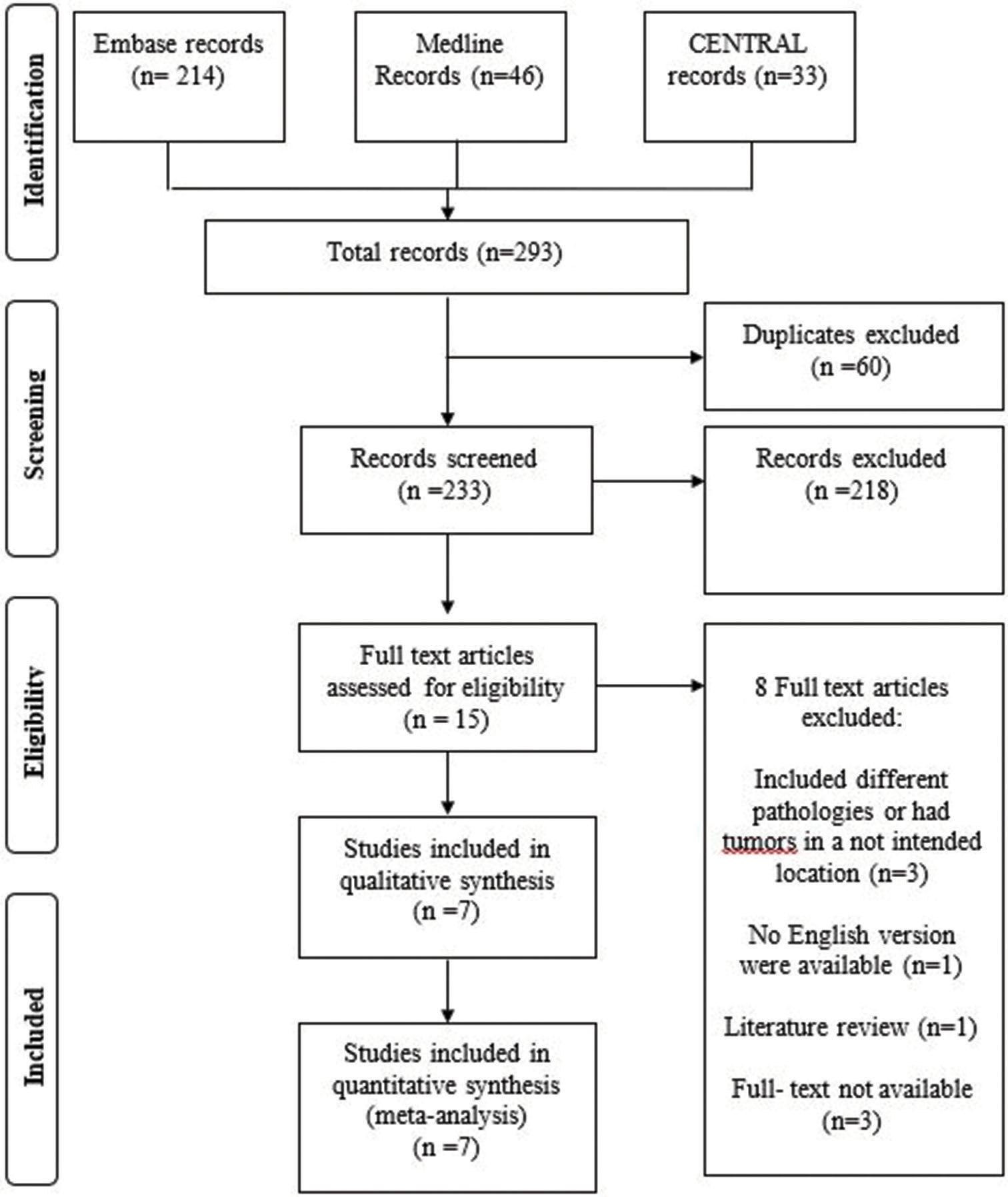
A total of 293 total articles were identified from our search. Sixty duplicate articles were excluded. 218 articles were further excluded after the title and abstract screening which left us with 15 eligible articles for full-text assessment. Ultimately, eight articles were excluded while seven articles met our inclusion criteria.[
Trial characteristics
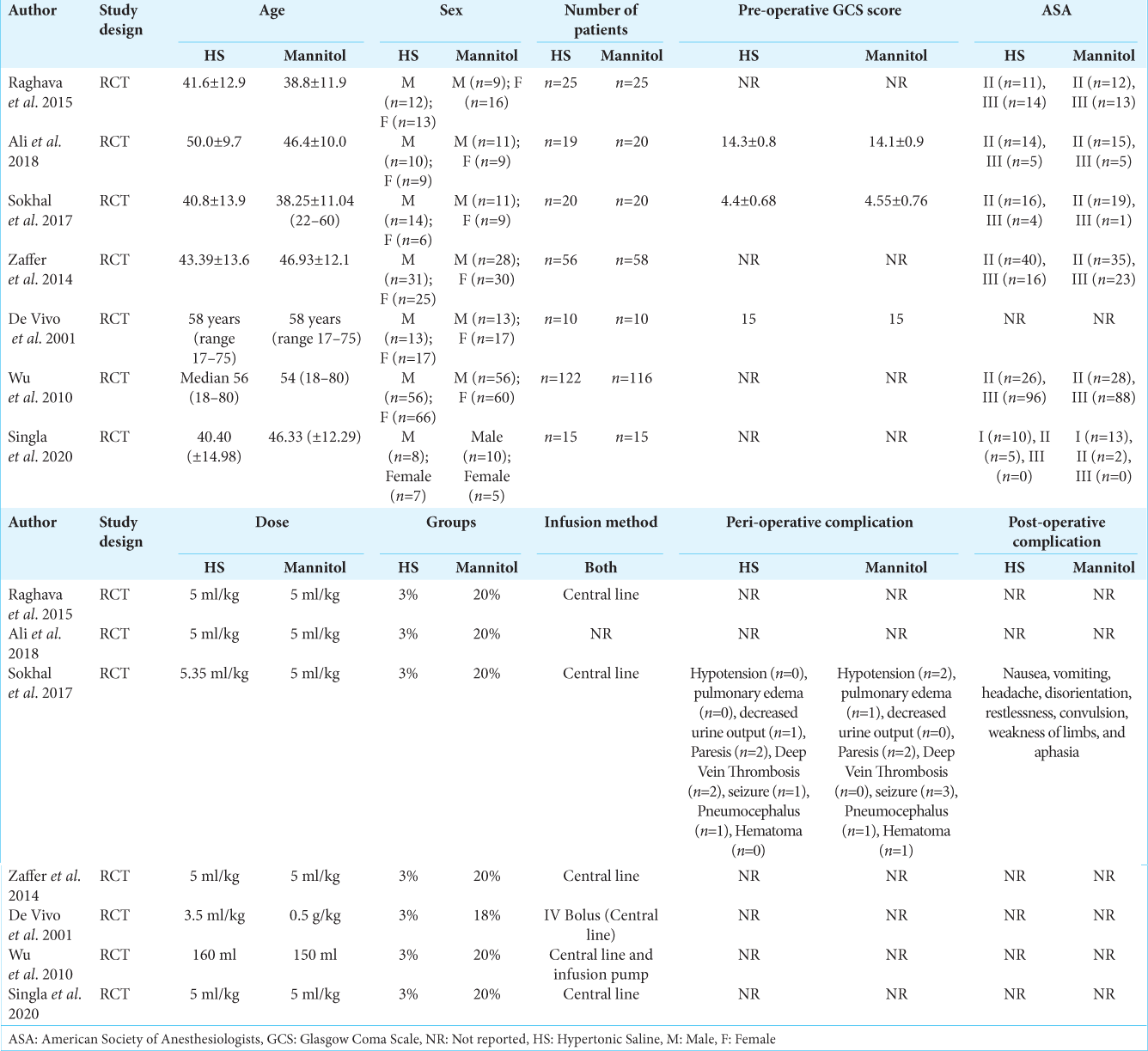
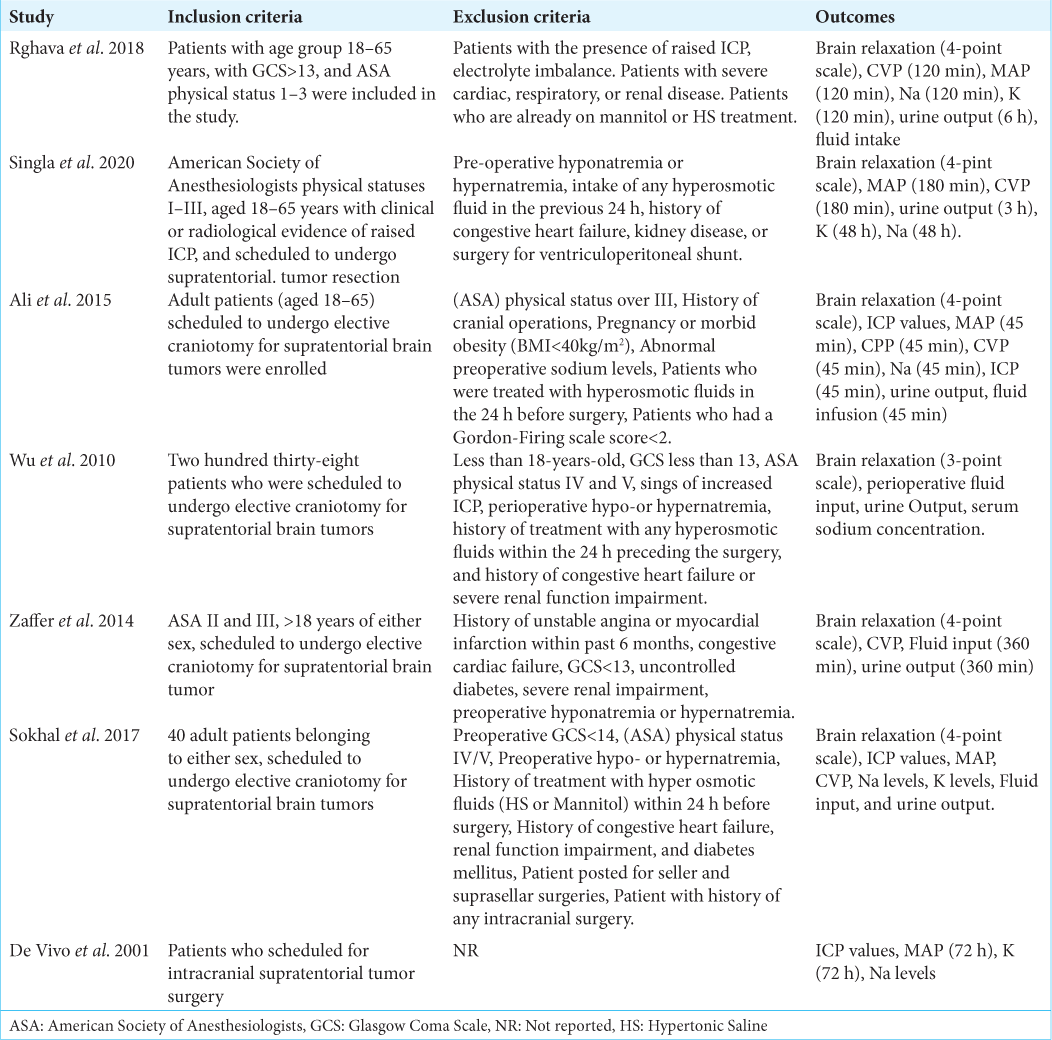
A total of 531 participants included in the seven studies. Of which 267 (50.2%) were allocated in the HS while 264 (49.7%) were allocated in the mannitol group. Of 531 participants, 282 (53.1%) were male and 249 (46.8%) were females with mean ages ranges from 38.25 to 58-years-old. The details of study characteristics are shown in [
Risk of bias assessment
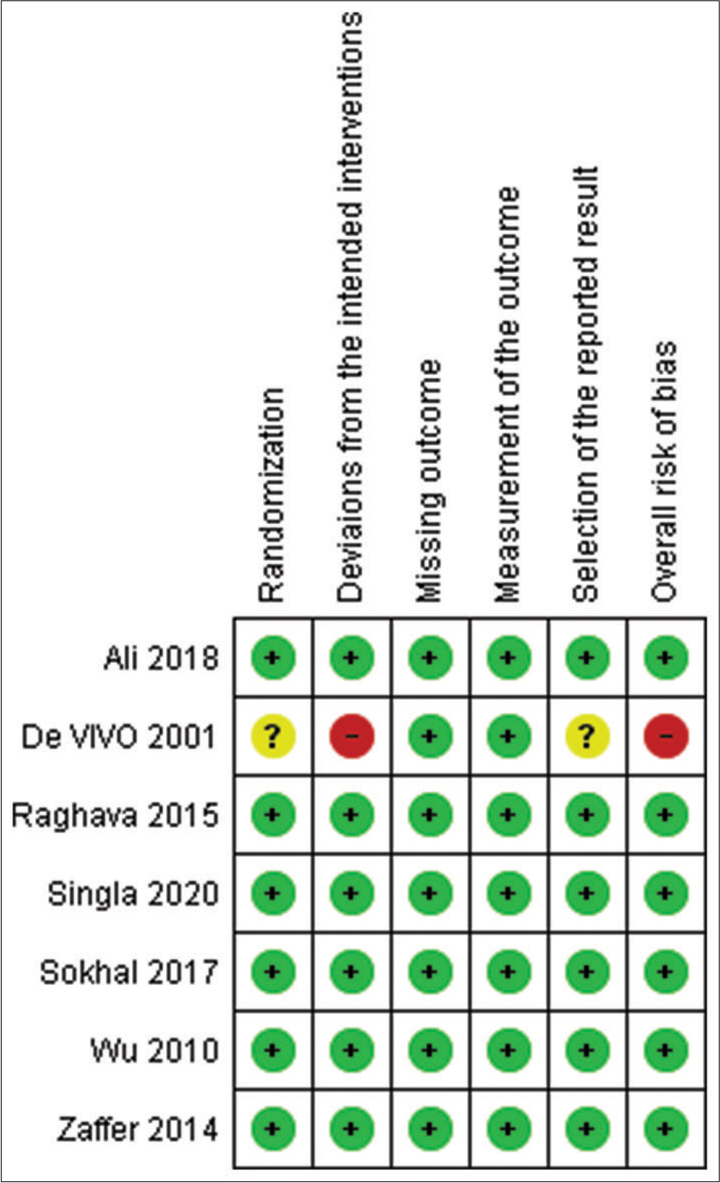
Of the seven studies, only one had an overall high risk of bias[
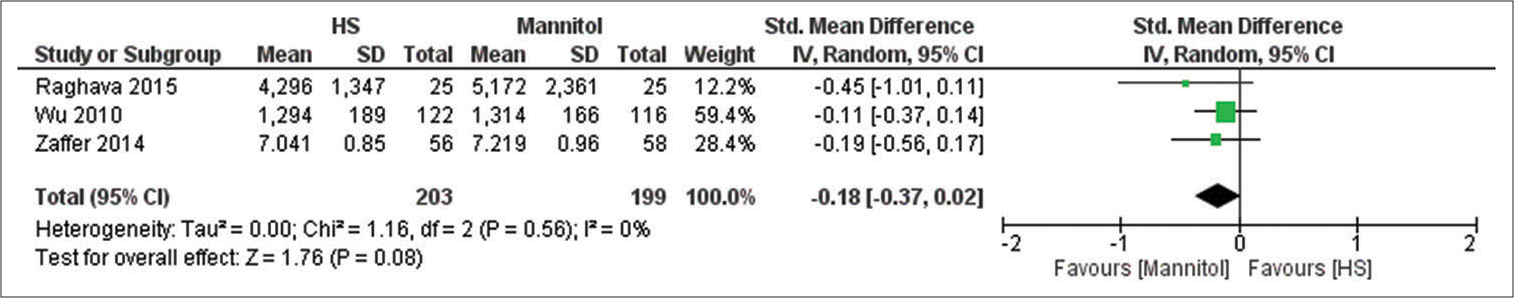
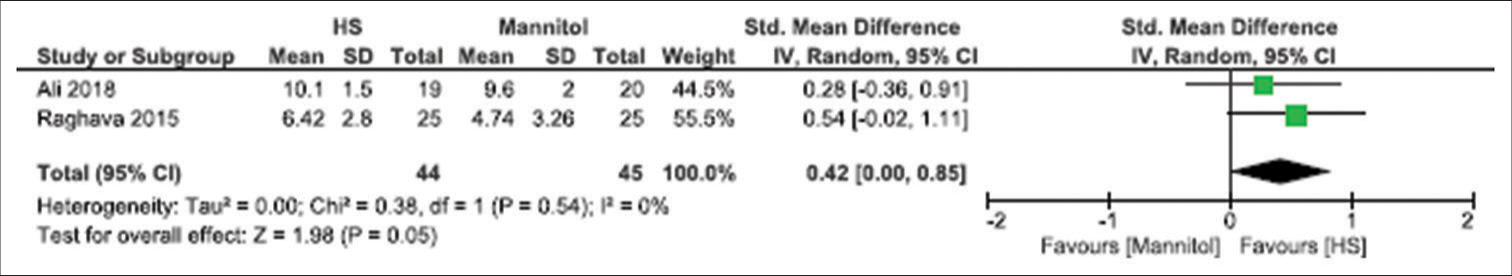
Brain relaxation (4-point scale)
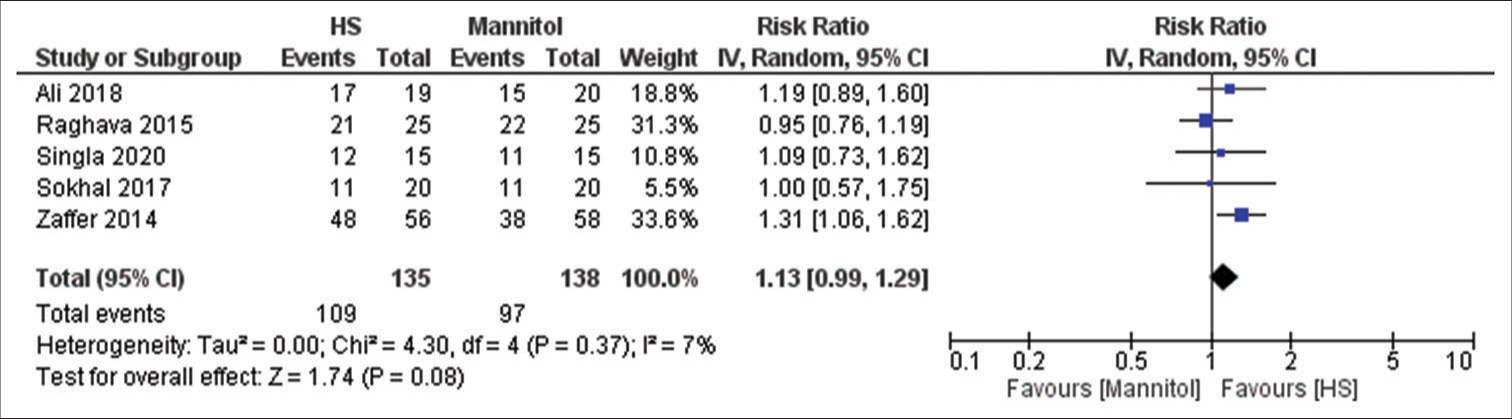
Five studies have reported this outcome with a sample size of 273 participants.[
ICP
Only one study has reported the exact value of the outcome[
Mean arterial pressure
Five studies have reported this outcome.[
CVP
All studies have reported this outcome, but only two studies have reported that exact value while the rest only reported it using figures.[
Perioperative fluid input
Fluid input has been reported by three studies with a sample size of 402 participants.[
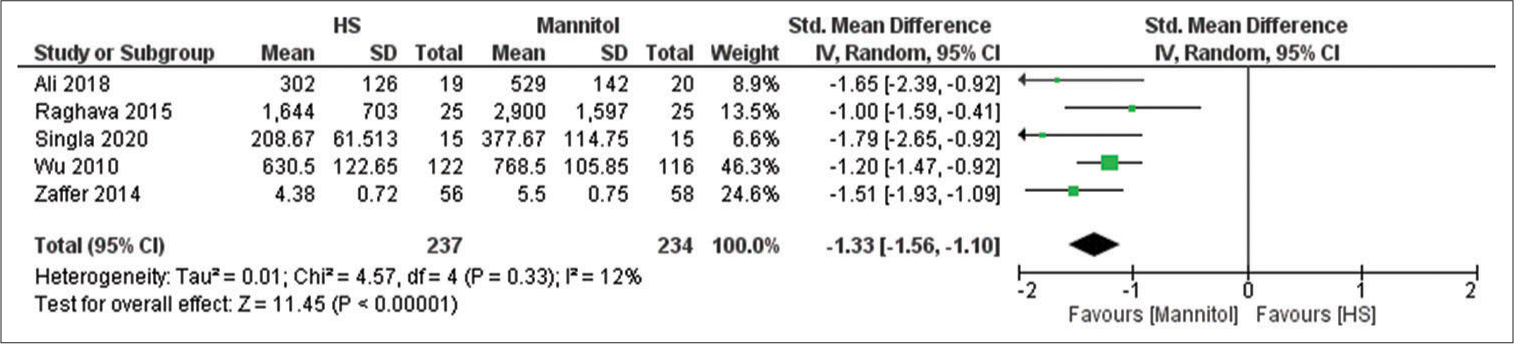
Urine output
Five studies reported the urine output with a sample size of 471 participants.[
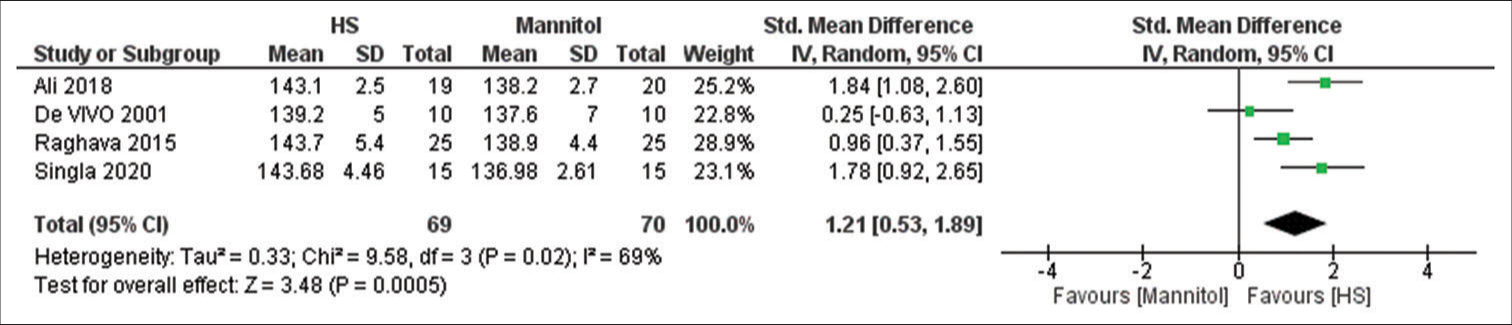
Na+ levels
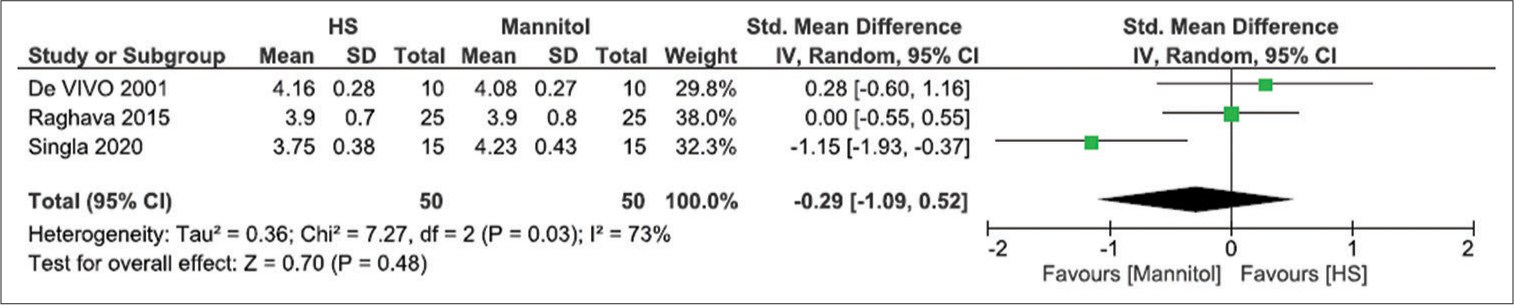
Four studies have reported this outcome with a sample size of 139 participants.[
K+ levels
Three studies have reported the outcome with a sample size of 100 participants.[
DISCUSSION
We aimed in this systematic review and meta-analysis to evaluate the efficacy of HS and mannitol in producing a satisfactory brain relaxation in patients undergoing supratentorial tumors craniotomies. Our review provided a high level of evidence since we included well-designed RCTs. Our review focused only on supratentorial tumors craniotomies rather than different brain pathologies. The difference between brain tumors and other brain pathologies is that brain tumors are space-occupying lesions and can induce peritumoral edema. The increase in ICP from brain tumors depends on different tumor factors, such as histological grade, and the degree of brain invasion. These factors are associated with a defective BBB and compromised venous outflow.[
Our study showed that HS has a higher tendency to produce better brain relaxation scores; however, our results were not statistically significant which contradict previous systematic review and meta-analysis done on the same issue. Fang et al. and Shao et al. concluded that HS have significantly better odds in producing a good brain relaxation score when compared to mannitol.[
We demonstrated in this review that HS was associated with significantly higher Na+ levels whereas mannitol was associated with significantly higher urine output. The increase in serum Na+ will increase serum osmolarity leading to activation of osmo receptors in the hypothalamus which activates the release of antidiuretic hormone contributing to fewer diuretic effects of HS.[
Our review found that HS was associated with non-significantly higher levels of CVP. This is consistent with the previous systematic reviews. Fang et al. concluded the mannitol significantly reduced CVP, but with comparable MAP in patients undergoing neurosurgical procedures for different brain pathologies. It is logical to think that this result may be similar in patients undergoing craniotomies for supratentorial tumors. In fact, this evidence is supported by many RCTs in craniotomies for supratentorial tumors not included in our meta-analysis.[
We acknowledge that our systematic review and meta-analysis had some limitations. First, our main outcome was subjectively assessed across all studies. Second, many studies have only reported their results using figures which limited our meta-analysis in different outcomes. Third, brain relaxation can be affected by different anesthesiologist measures. Finally, we were limited by the small sample size and the small number of studies.
CONCLUSION
Both HS and mannitol can produce a satisfactory brain relaxation with a non-statistically significant tendency for HS to achieve better relaxation scores. Mannitol was associated with higher diuretic effect whereas HS was associated with higher Na+ levels. Lower perioperative fluid input and higher CVP were found to be associated with HS. We recommend the use of HS in favor of mannitol in patients undergoing supratentorial tumor surgeries that do not demonstrate any electrolytes disturbances as HS has an edge over mannitol also HS can maintain an acceptable hemodynamics stability. Further high quality RCTs with a large sample size, an objective measurement of ICP, and standardized anesthesiologist measures are required to provide a more satisfactory conclusion.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY
References
1. Ali A, Tetik A, Sabanci PA, Altun D, Sivrikoz N, Abdullah T. Comparison of 3% hypertonic saline and 20% mannitol for reducing intracranial pressure in patients undergoing supratentorial brain tumor surgery: A randomized, double-blind clinical trial. J Neurosurg Anesthesiol. 2018. 30: 171-8
2. Berl T, Robertson G.editors. Pathophysiology of Water Metabolism. Philadelphia, PA: Saunders; 2000. p.
3. De Vivo P, Del Gaudio A, Ciritella P, Puopolo M, Chiarotti F, Mastronardi E. Hypertonic saline solution: A safe alternative to mannitol 18% in neurosurgery. Minerva Anestesiol. 2001. 67: 603-11
4. Dostal P, Dostalova V, Schreiberova J, Tyll T, Habalova J, Cerny V. A comparison of equivolume, equiosmolar solutions of hypertonic saline and mannitol for brain relaxation in patients undergoing elective intracranial tumor surgery: A randomized clinical trial. J Neurosurg Anesthesiol. 2015. 27: 51-6
5. Fang J, Yang Y, Wang W, Liu Y, An T, Zou M. Comparison of equiosmolar hypertonic saline and mannitol for brain relaxation during craniotomies: A meta-analysis of randomized controlled trials. Neurosurg Rev. 2018. 41: 945-56
6. Hans P, Bonhomme V. Why we still use intravenous drugs as the basic regimen for neurosurgical anaesthesia. Curr Opin Anaesthesiol. 2006. 19: 498-503
7. Hernández-Palazón J, Fuentes-García D, Doménech-Asensi P, Piqueras-Pérez C, Falcón-Araña L, Burguillos-López S. A comparison of equivolume, equiosmolar solutions of hypertonic saline and mannitol for brain relaxation during elective supratentorial craniotomy. Br J Neurosurg. 2016. 30: 70-5
8. Hinson HE, Stein D, Sheth KN. Hypertonic saline and mannitol therapy in critical care neurology. J Intensive Care Med. 2013. 28: 3-11
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009. 151: W65-94
10. Malik ZA, Mir SA, Naqash IA, Sofi KP, Wani AA. A prospective, randomized, double blind study to compare the effects of equiosmolar solutions of 3% hypertonic saline and 20% mannitol on reduction of brain-bulk during elective craniotomy for supratentorial brain tumor resection. Anesth Essays Res. 2014. 8: 388-92
11. Ogden AT, Mayer SA, Connolly ES. Hyperosmolar agents in neurosurgical practice: The evolving role of hypertonic saline. Neurosurgery. 2005. 57: 207-15
12. Raghava A, Bidkar PU, Prakash MV, Hemavathy B. Comparison of equiosmolar concentrations of hypertonic saline and mannitol for intraoperative lax brain in patients undergoing craniotomy. Surg Neurol Int. 2015. 6: 73
13. Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007. 107: 697-704
14. Schwimmbeck F, Voellger B, Chappell D, Eberhart L. Hypertonic saline versus mannitol for traumatic brain injury: A systematic review and meta-analysis with trial sequential analysis. J Neurosurg Anesthesiol. 2021. 33: 10-20
15. Shao L, Hong F, Zou Y, Hao X, Hou H, Tian M. Hypertonic saline for brain relaxation and intracranial pressure in patients undergoing neurosurgical procedures: A meta-analysis of randomized controlled trials. PLoS One. 2015. 10: e0117314
16. Singla A, Mathew PJ, Jangra K, Gupta SK, Soni SL. A comparison of hypertonic saline and mannitol on intraoperative brain relaxation in patients with raised intracranial pressure during supratentorial tumors resection: A randomized control trial. Neurol India. 2020. 68: 141-5
17. Sokhal N, Rath GP, Chaturvedi A, Singh M, Dash HH. Comparison of 20% mannitol and 3% hypertonic saline on intracranial pressure and systemic hemodynamics. J Clin Neurosci. 2017. 42: 148-54
18. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019. 366: l4898
19. Tenny S, Patel R, Thorell W.editors. Mannitol. Treasure Island, FL: StatPearls Publishing; 2021. p.
20. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014. 14: 135
21. Witherspoon B, Ashby NE. The use of mannitol and hypertonic saline therapies in patients with elevated intracranial pressure: A review of the evidence. Nurs Clin North Am. 2017. 52: 249-60
22. Wu CT, Chen LC, Kuo CP, Ju DT, Borel CO, Cherng CH. A comparison of 3% hypertonic saline and mannitol for brain relaxation during elective supratentorial brain tumor surgery. Anesth Analg. 2010. 110: 903-7
23. Ziai WC, Toung TJ, Bhardwaj A. Hypertonic saline: First-line therapy for cerebral edema?. J Neurol Sci. 2007. 261: 157-66