- Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States.
- Department of Otolaryngology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States.
- Department of Center for Image-Guided Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States.
- Center for Skull Base Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States.
Correspondence Address:
Hansen Deng, Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States.
DOI:10.25259/SNI_821_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hansen Deng1, Michael M. McDowell1, Zachary C. Gersey1, Hussam Abou-Al-Shaar1, Carl H. Snyderman2, Georgios A. Zenonos1, L. Dade Lunsford3, Paul A. Gardner4. Esthesioneuroblastoma with recurrent dural metastases: Long-term multimodality treatment and considerations. 08-Dec-2021;12:606
How to cite this URL: Hansen Deng1, Michael M. McDowell1, Zachary C. Gersey1, Hussam Abou-Al-Shaar1, Carl H. Snyderman2, Georgios A. Zenonos1, L. Dade Lunsford3, Paul A. Gardner4. Esthesioneuroblastoma with recurrent dural metastases: Long-term multimodality treatment and considerations. 08-Dec-2021;12:606. Available from: https://surgicalneurologyint.com/surgicalint-articles/11266/
Abstract
Background: Esthesioneuroblastoma (ENB) is a rare malignant disease and treatment protocols have not been standardized, varying widely by disease course and institutional practices. Management typically includes wide local excision through open or endoscopic resection, followed by radiotherapy, chemotherapy, and stereotactic radiosurgery. Tumor control can differ on a case-by-case basis. Herein, the complex management of a rare case of recurrent disease with multiple dural metastases is presented.
Case Description: A 60-year-old patient was diagnosed with ENB after presenting with anosmia and epistaxis. The patient underwent combined endonasal and transfrontal sinus craniofacial resection, followed by proton beam radiation therapy and chemotherapy. Subsequently, he developed a total of 25 dural metastases that were controlled with repeated Gamma Knife Radiosurgery (GKRS). In spite of post-treatment course that was complicated by radiation necrosis and local vasculopathy, the patient made significant recovery to functional baseline.
Conclusion: The management of ENB entails multimodality and multidisciplinary care, which can help patients obtain disease control and long-term survival. Recurrent ENB dural metastases can behave as oligometastatic disease manageable with aggressive focal GKRS. As prognosis continues to improve, chronic treatment effects of radiation in such cases should be taken into consideration.
Keywords: Esthesioneuroblastoma, Gamma knife, Kadish, Radiotherapy, Recurrence
INTRODUCTION
First reported in 1924, esthesioneuroblastoma (ENB), also referred to as olfactory neuroblastoma, is a rare, malignant nasal tumor that arises from the olfactory neuroepithelium with an incidence of 0.4/1,000,000 individuals.[
The first report of endoscopically assisted resection with bi-coronal craniotomy was in 1997,[
While postoperative RT does independently predict better disease-specific survival,[
CASE REPORT
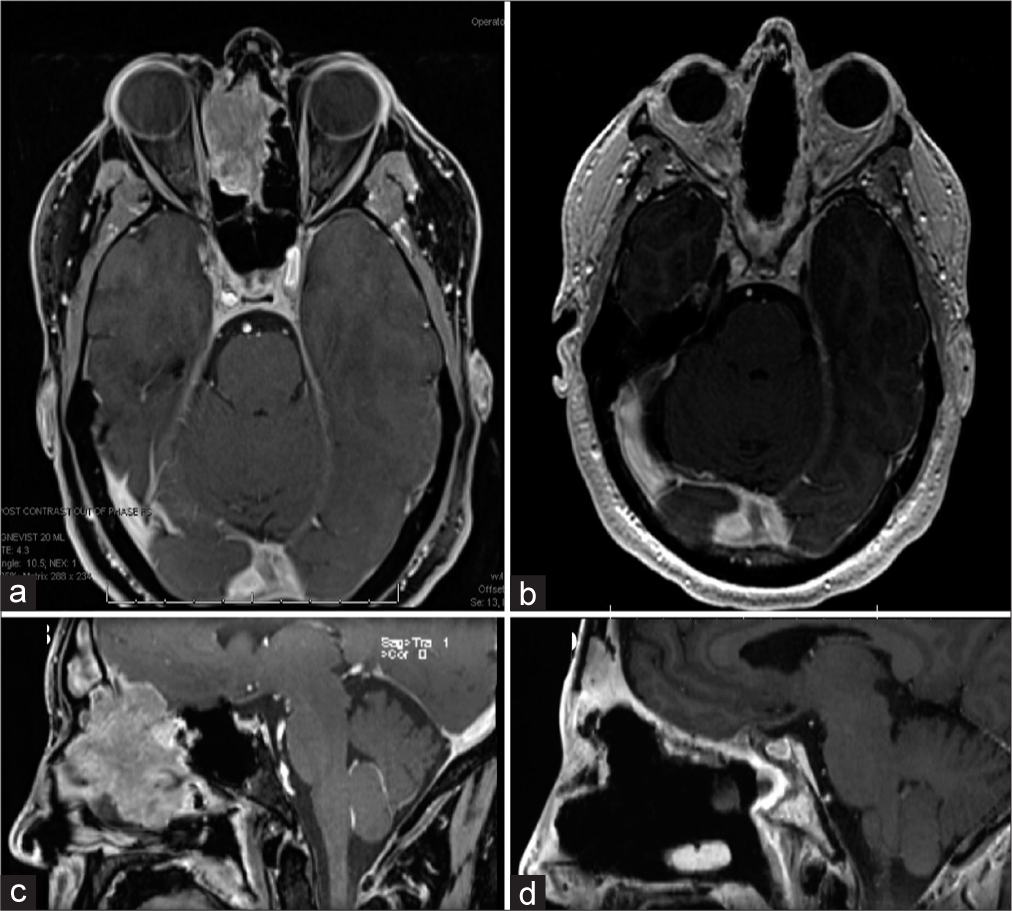
The patient was a 60-year-old right-handed man who initially presented with 5 months of progressive loss of sense of smell, right-sided epistaxis, and excessive tearing of the right eye. Magnetic resonance imaging showed evidence of Kadish C disease [
Following resection, the patient received fractionated proton beam radiotherapy at an outside institution and concomitant cisplatin. The patient experienced anosmia and a partial right sixth cranial nerve palsy. Five years later, the patient developed meningeal metastases. He received GKRS for a total of 12 dural-based metastases [
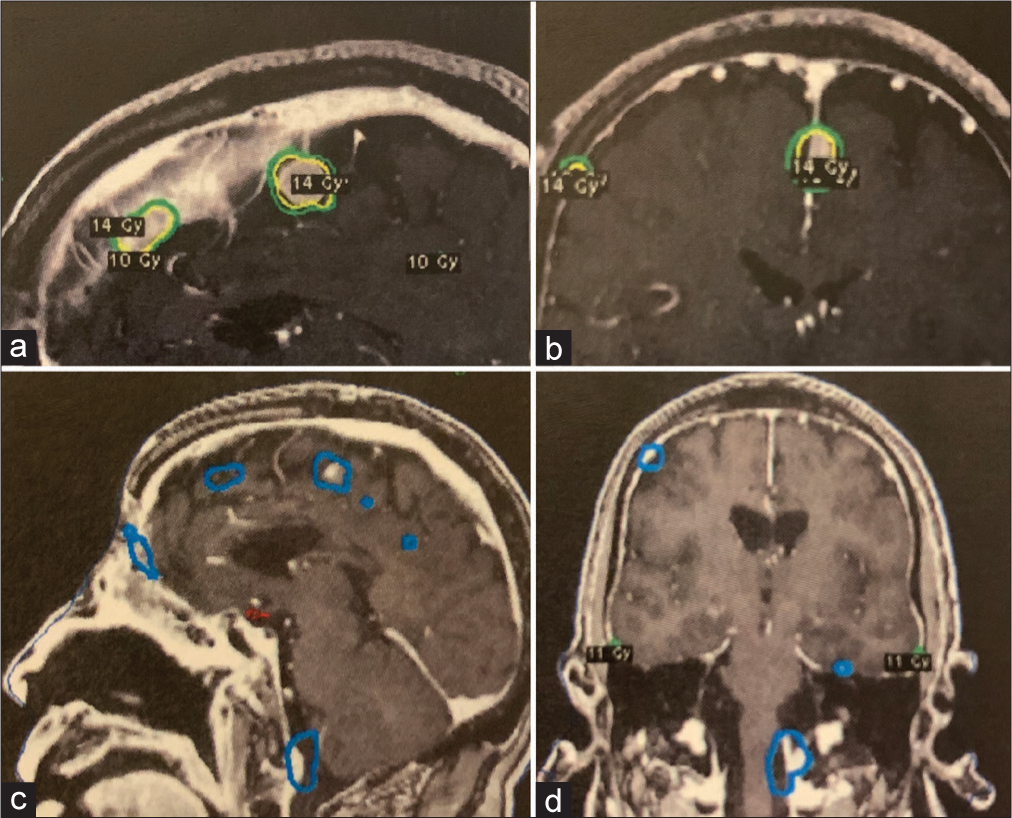
Figure 2:
Brain fast spoiled gradient echo sequences with contrast depicting multiple meningeal metastases that are targeted during the second Gamma Knife Radiosurgery (GKRS) treatment, with the margin dose labeled in yellow on the sagittal (a) and coronal (b) planes on the day of GKRS. Interval follow-up imaging after GKRS showing treatment response and decrease in tumor volume on sagittal (c) and coronal (d) sequences.
Six months post-treatment, the patient returned with headache and personality changes. There were radiographic findings of radiation necrosis and pericranium flap breakdown [
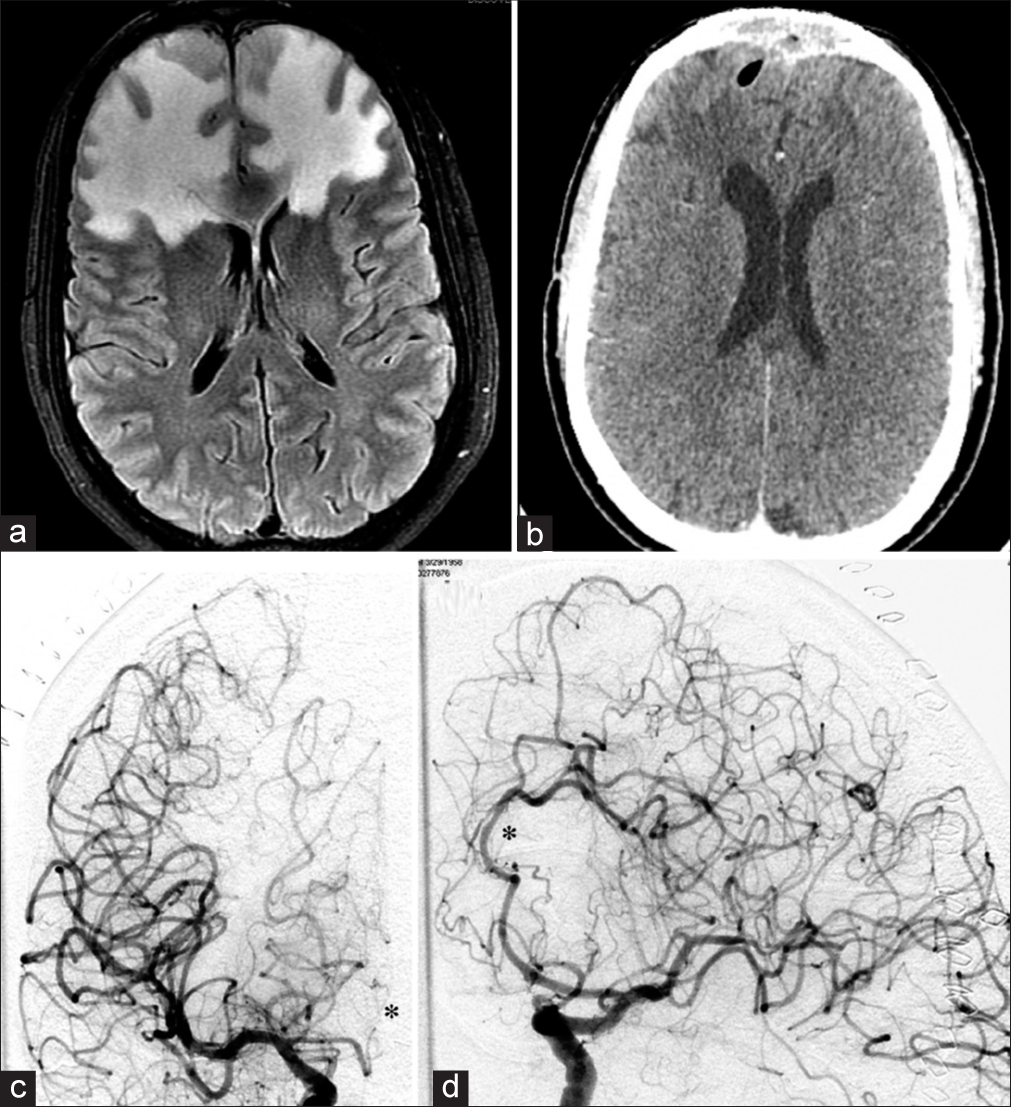
Figure 3:
MRI brain FLAIR sequence demonstrating pericranium radiation necrosis of the frontal lobes (a), and CT head demonstrating flap compromise with intracerebral air (b). DSA performed postoperatively that demonstrates on the right coronal ICA injection an atretic ACA that fills sluggishly to the mid A2 segment where it abruptly stops (c). The left coronal ICA injection reveals irregularities in the distal branches of the A2 and A3 segments that are consistent with radiation changes (d).
For the reconstruction of the anterior cranial fossa, the plastic surgery team harvested vastus lateralis muscle free flap for anastomosis with superficial temporal artery. An external ventricular drain was placed. The vastus lateralis flap demonstrated good vascular supply and appeared healthy on follow-up endoscopy. The patient had postoperative left leg weakness that improved significantly with rehabilitation. Following debridement, the patient received two more treatments of GKRS for new dural metastases on surveillance imaging. The first treatment occurred 9 months later, to six tumors located in the left sigmoid, left tentorium, left lateral temporal, right lateral temporal, left parietal, and left posterior parietal. A margin dose of 14 Gy ranging in 50–80% isodose was given. Five months later, a new lesion involving the left jugular tubercle was detected and GKRS was performed using a margin dose of 15 Gy. Aside from intermittent cognitive complaints, the patient remained functionally independent and active with no new metastases 24-months following the last treatment of GKRS.
DISCUSSION
ENB is a rare and slow-growing malignant neoplasm that comprises 2% of all sinonasal tract tumors. Nasal obstruction from the mass is the most common complaint from the patient. Other symptoms can be from the invasion of adjacent structures including the cribriform plate, orbit, eustachian tube, and frontal sinus. Early recognition is important to allow for the greatest potential for aggressive gross total resection in addition to adjuvant radiation for local control. Our patient had Kadish stage C progression with a histologically low-grade ENB. In a previous institutional report of 20 Kadish C patients, 45% of subjects had low-grade tumor and their 5-year disease-free survival was 75% following surgery with adjuvant therapy.[
Radiotherapy or stereotactic radiosurgery (SRS) has been an integral modality of head and neck cancer therapy. While it is important to control recurrence as was the case in our patient, radiation is not without long-term effects. In surgical patients, adjuvant radiation can disrupt early inflammatory and proliferative wound healing stages, as well as the late remodeling phase important for tissue reconstitution. The effect of disorganized collagen deposition by fibroblasts in irradiated tissue can accumulate over the course of years, and dose-dependent gamma exposures in mice have been shown to possibly decrease collagen and vascular densities.[
Combined surgery and radiation recently have become standards of practice in patients with this rare disease. Radiation options vary by institutional preference and the availability of in-hospital technology. In cases that require repeated treatment as well as eloquent regions, conformal SRS in single treatments can be performed with minimal toxicity to surrounding tissue. Reducing fall off dosage to surrounding tissue is critical. Given that the median survival is 7.2 years,[
Radiation-induced vessel changes are an important consideration, especially in cases that need reoperation. Aneurysms and vasculopathy can occur within 10 years post-radiation, as was the case here in the setting of reirradiation. The pathogenesis is likely due to endothelial injury, with hemodynamic flow and structural weakness that lead to aneurysmal formation.[
It is important to note the effectiveness of radiosurgery in management of the multiple metastases from ENB. There is not established chemotherapy protocol for recurrent disease leaving surgical resection (not feasible for multiple lesions in morbid locations) or radiation as options. Targeted radiosurgery would maximize tumor control and minimize secondary effects. The development of new dural metastases can be addressed with repeated GKRS. It appears that recurrent ENB dural metastases can behave as oligometastatic disease manageable with aggressive focal therapy.
CONCLUSION
As survival for patients with ENB improves through multimodality treatment, long-term treatment effects associated with recurrent disease have not been well reported in the literature. This is the first such report of a patient with extensive dural and skull base metastases for which targeted GKRS through single treatments were performed effectively for tumor control. Management relies on close radiographic monitoring and repeated SRS. Chronic cerebrovascular changes in prior radiation fields should be identified early on.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chin LS, Lazio BE, Biggins T, Amin P. Acute complications following gamma knife radiosurgery are rare. Surg Neurol. 2000. 53: 498-502
2. Devaiah AK, Larsen C, Tawfik O, O’Boynick P, Hoover LA. Esthesioneuroblastoma: Endoscopic nasal and anterior craniotomy resection. Laryngoscope. 2003. 113: 2086-90
3. Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: A meta-analysis and review. Lancet Oncol. 2001. 2: 683-90
4. Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005. 44: 13-22
5. Flickinger JC, Kondziolka D, Dade Lunsford L, Kassam A, Phuong LK, Liscak R. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Int J Radiat Oncol Biol Phys. 2000. 46: 1143-8
6. Jagetia GC, Rajanikant GK, Rao SK. Evaluation of the effect of ascorbic acid treatment on wound healing in mice exposed to different doses of fractionated gamma radiation. Radiat Res. 2003. 159: 371-80
7. Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: A population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007. 133: 276-80
8. Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976. 37: 1571-6
9. Kaur G, Kane AJ, Sughrue ME, Madden M, Oh MC, Sun MZ. The prognostic implications of Hyam’s subtype for patients with Kadish stage C esthesioneuroblastoma. J Clin Neurosci. 2013. 20: 281-6
10. Kellner CP, McDowell MM, Connolly ES, Sisti MB, Lavine SD. Late onset aneurysm development following radiosurgical obliteration of a cerebellopontine angle meningioma. J Neurointerv Surg. 2015. 7: e21
11. Nanney AD, El Tecle NE, El Ahmadieh TY, Daou MR, Bit Ivan EN, Marymont MH. Intracranial aneurysms in previously irradiated fields: Literature review and case report. World Neurosurg. 2014. 81: 511-9
12. Nakamura JL, Verhey LJ, Smith V, Petti PL, Lamborn KR, Larson DA. Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys. 2001. 51: 1313-9
13. Sekhar LN, Heros RC. Origin, growth, and rupture of saccular aneurysms: A review. Neurosurgery. 1981. 8: 248-60
14. Tajudeen BA, Arshi A, Suh JD, St John M, Wang MB. Importance of tumor grade in esthesioneuroblastoma survival: A population-based analysis. JAMA Otolaryngol Head Neck Surg. 2014. 140: 1124-9
15. Theilgaard SA, Buchwald C, Ingeholm P, Larsen SK, Eriksen JG, Hansen HS. Esthesioneuroblastoma: A Danish demographic study of 40 patients registered between 1978 and 2000. Acta Otolaryngol. 2003. 123: 433-9
16. Thompson LD. Olfactory neuroblastoma. Head Neck Pathol. 2009. 3: 252-9
17. Umana GE, Raudino G, Alberio N, Inserra F, Giovinazzo G, Fricia M. Slit-like hypertensive hydrocephalus: Report of a late, complex, and multifactorial complication in an oncologic patient. Surg Neurol Int. 2020. 11: 219
18. van Gompel JJ, Link MJ, Sheehan JP, Xu Z, Mathieu D, Kano H. Radiosurgery is an effective treatment for recurrent esthesioneuroblastoma: A multicenter study. J Neurol Surg B Skull Base. 2014. 75: 409-14
19. Yuen AP, Fung CF, Hung KN. Endoscopic cranionasal resection of anterior skull base tumor. Am J Otolaryngol. 1997. 18: 431-3