- Neurosurgery Unit, Fondazione IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) Policlinico San Matteo, Pavia, Italy
- Department of Internal Medicine and Medical Therapeutics, University of Pavia, Pavia, Italy
- Internal Medicine Unit, Fondazione IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) Policlinico San Matteo, Pavia, Italy
- Clinical Department, National Center for Oncological Hadrontherapy (CNAO), Pavia, Italy
- Neurosurgery Unit, Ospedale Fatebenefratelli e Oftalmico, Azienda Socio Sanitaria Territoriale (ASST) Fatebenefratelli Sacco, Milan, Italy
- Department of Experimental Oncology, European Institute of Oncology IRCCS, Milan, Italy.
Correspondence Address:
Giada Todeschini, Department of Internal Medicine and Medical Therapeutics, University of Pavia, Pavia, Italy.
DOI:10.25259/SNI_41_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Cesare Zoia1, Giada Todeschini2, Elisabetta Lovati3, Pietro Lucotti3, Alberto Iannalfi4, Daniele Bongetta5, Antonio Di Sabatino3, Giulia Riva4, Iacopo Cavallo4,6, Ester Orlandi4, Giannantonio Spena1. Evaluation of endocrinological sequelae following particle therapy performed on anterior skull base lesions in the adult population. 18-Aug-2023;14:293
How to cite this URL: Cesare Zoia1, Giada Todeschini2, Elisabetta Lovati3, Pietro Lucotti3, Alberto Iannalfi4, Daniele Bongetta5, Antonio Di Sabatino3, Giulia Riva4, Iacopo Cavallo4,6, Ester Orlandi4, Giannantonio Spena1. Evaluation of endocrinological sequelae following particle therapy performed on anterior skull base lesions in the adult population. 18-Aug-2023;14:293. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12504
Abstract
Background: Radiotherapy has increasingly assumed a central role in the multidisciplinary treatment of skull base lesions. Unfortunately, it is often burdened by relevant radio-induced damage to the pituitary function and the surrounding structures and systems. Patients who were treated with radiotherapy around the sellar region especially have a high risk of developing radio-induced hypopituitarism. Particle therapy has the potential advantage of delivering a higher radiation dose to the target while potentially sparing the sellar region and pituitary function. The aim of this study is to evaluate the pituitary function in adult patients who have undergone hadron therapy for anterior skull base lesions involving or surrounding the pituitary gland.
Methods: This is a retrospective, observational, and noncontrolled study. We evaluated pituitary and peripheral hormone levels in all patients referring to National Center for Oncological Hadrontherapy, Pavia, Italy for anterior skull base tumors. Furthermore, we performed a magnetic resonance imaging for every follow-up to evaluate potential tumoral growth.
Results: We evaluated 32 patients with different tumoral lesions with a mean follow-up of 27.9 months. The mean hadron therapy (HT) dose was 60 ± 14 Gray, with a mean dose per fraction of 2.3 ± 2.1 Gray. Six patients were treated with carbon ions and 26 with protons. Pituitary hormone alteration of some kind was reported for six patients. No patient experienced unexpected severe adverse events related to particle therapy.
Conclusion: Particle radiotherapy performed on anterior skull base lesions has proved to cause limited damage to pituitary function in the adult population.
Keywords: Hadron therapy, Hormones, Particle therapy, Pituitary, Skull base
INTRODUCTION
Radiotherapy has increasingly assumed a central role in the multidisciplinary treatment of skull base lesions. However, when targeting anterior skull base lesions, it could cause relevant radio-induced damage to the pituitary function and the surrounding structures.[
Particle radiotherapy (PT), on the other hand, has the potential advantage of delivering a higher radiation dose to the target while minimizing the damage toward the sellar region.[
Studies have also been carried out to identify a dosage tolerance threshold, beyond which more serious endocrinological complications arise – mostly in terms of pituitary function deficits.[
Patients referred for PT for anterior skull base lesions represent an ideal population to evaluate endocrinological toxicity on radio-induced pituitary dysfunction.
For this reason, the aim of this study is to evaluate the pituitary function in adult patients who have undergone PT for anterior skull base lesions involving or surrounding the pituitary gland.
The management of sellar lesions requires a multidisciplinary approach.[
MATERIALS AND METHODS
For this study, we enrolled 32 adult patients with different tumors involving the skull base and sellar region. The average follow-up time was 27.9 months.
Among these patients, 22 had already undergone neurosurgery, two had undergone conventional radiotherapy in addition to surgery, and eight had never approached the lesion with any treatment.
The treated tumors were the following: One pituitary macroadenoma, 16 meningiomas, two craniopharyngiomas, three carcinomas (two undifferentiated and 1 cystic), one mucosal melanoma, one glioma, two chondrosarcomas of the clivus, and six chordomas of the clivus.
All patients were treated with a multidisciplinary approach. Radiological, endocrinological, and neurosurgical evaluations found place both before and after the treatment, with preestablished follow-up times (at 3, 6, 9, 12, 18, and 24 months, then annually). For all patients, venous blood was collected to test for hormonal levels, together with urine samples. Blood hormone levels and 24-h urinary cortisol were re-evaluated during each follow-up.
Out of these patients, six were treated with carbon ions and 26 with protons [
Hormonal alterations are reported in
During each follow-up, a magnetic resonance imaging (MRI) was also performed to evaluate the lesion and the possible infiltration of surrounding structures.
In addition, patients were requested to fill in a questionnaire concerning potential side effects related to particle therapy (mucositis, skin reactions, deficit of cranial nerves V and VIII, and memory deficit) and irreversible side effects previously caused by the injury (visual impairment and headache). Toxicity levels were evaluated with Common Terminology Criteria for Adverse Events scale, version 5.0.[
Ophthalmological visits were also performed to assess pretreatment and posttreatment ophthalmological balance.[
All patients provided written informed consent to take part in the study.
Statistical analysis was performed using Student’s t-test[
RESULTS
Results in terms of quality of life and loss in terms of autonomy (according to Karnofsky Performance Scale [KPS] index), headache (assessed on the basis of Visual Analogue Scale pain scale), visual impairment, cranial nerve deficits (c.n. V and VIII), memory deficits, skin changes, toxicity detected during last follow-up, and hormonal changes are summarized in
The mean dose of administered particle therapy was 60 ± 14 Gray(RBE) (GyRBE), with a mean dose per fraction of 2.3 ± 2.1 Gy.
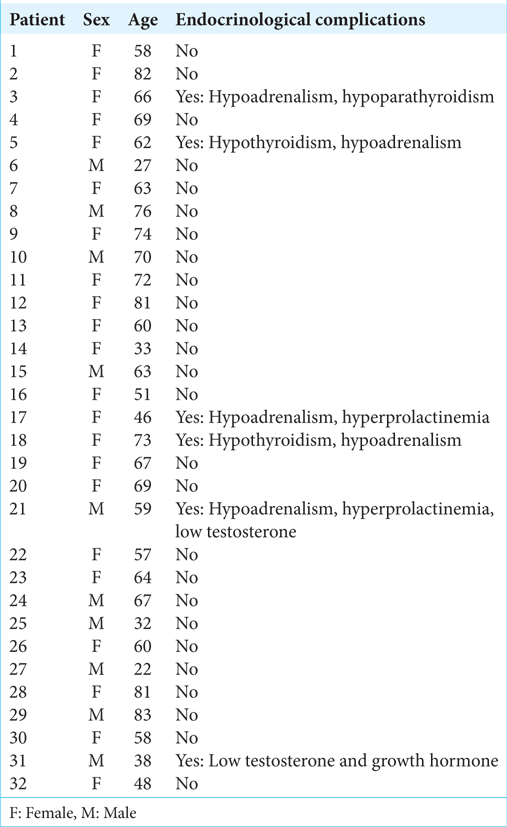
Out of 32 patients, 26 developed no hormonal alterations. Pituitary hormone alterations were reported for 6 patients (18.75%) [
Patients with hormonal changes were patients 3, 5, 17, 18, 21, and 31.
Patient 3 already suffered from central hypothyroidism (presumably caused by the tumor) and were undergoing thyroid replacement therapy. Following the treatment, however, the patient was also diagnosed with central adrenal insufficiency and primary hypoparathyroidism.
Patient 5 had to undergo a levothyroxine and glucocorticoid replacement therapy due to central hypothyroidism and adrenal insufficiency. Central hypothyroidism was already present before treatment, while adrenal insufficiency was discovered 6 months after the end of therapy.
Patient 17 was diagnosed with mild central adrenal insufficiency and hyperprolactinemia 6 months after completing of therapy, but no treatment was started. At the last follow-up, the values were back within range.
Patient 18 was diagnosed with central hypothyroidism and central adrenal insufficiency 2 years after completing therapy and started replacement therapy with cortisone acetate and levo-thyroxine.
Patient 21 showed a decrease in blood testosterone levels, together with an increase in prolactin, 5 years after the end of therapy (presumably a relapse). Furthermore, 6 years after the end of treatment, the patient was hospitalized for severe hypo-osmolar hyponatremia and diagnosed with severe central adrenal insufficiency. He then started replacement therapy with cortisone acetate.
Patient 31 showed a reduction in testosterone, as well as an increase in FSH and LH. For this reason, testosterone replacement therapy was started.
For the remaining 26 patients, no new hormonal alterations were detected and no replacement therapy was necessary (except for those who already showed hormonal alterations before PT).
Visual impairment (if present) remained stable compared to pretreatment, except in Patient 3, who experienced G4 – complete postactinic blindness.
Headache was reported as a side effect in six out of 32 patients (18.75%), with a maximum reported pain of 9/10 in Patient 7.
Some patients reported nerve deficits, particularly cranial nerve V and VIII (trigeminal and vestibular-cochlear). In 10 patients a trigeminal deficit was reported (31.25%), while a vestibulo-cochlear deficit was detected in 8 patients (25%). The maximum reported grade for both deficits was G2.
During follow-up visits, a possible memory deficit was also investigated. This was reported in 6 patients (18.75%), with maximum grade G3. Five patients also reported postactinic skin changes (15.6%), with a maximum grade of G2.
Case illustration patient 14
To better understand the diagnostic and therapeutic process of the patients enrolled in this study, we report the case of a patient belonging to the cohort.
The patient was a previously healthy 30-year-old female in the third trimester of her first pregnancy. She suffered no pregnancy-related complications except for gestational hypothyroidism, which was treated with levo-thyroxine. The patient was brought to the Neurosurgeons’ attention because of new-onset diplopia and left periorbital neuralgia. For this reason, she underwent an MRI and an eye examination. The MRI showed an expansive extra-axial lesion of the cavernous sinus (25 × 17 × 21 mm) that wrapped the carotid siphon, markedly reducing its caliber [
Due to this finding, a cesarean section was performed at the 35 weeks of gestation. The patient was also referred for particle therapy treatment, since she was showing symptoms.
The proton treatment was carried out from February 27, 2017 to March 15, 2017, with a total dose of 55.8 GyRBE (1.8 GyRBE/fraction, 31 fractions, 5 fractions/week) and intensity-modulated proton therapy (IMPT) technique [
At the end of proton therapy, the patient’s conditions were good, except for slight skin erythema (G1 toxicity) with initial alopecia. She reported no visual disturbances or headaches. At discharge, pain according to Numeric Pain Rating scale was 0/10. No hormone replacement therapy was necessary.
During follow-up visits, the patient’s MRIs showed a clear reduction (27 × 10 × 21 mm) and subsequent stability of the mass [
Therefore, we can state that the patient’s hormonal alterations were not caused by radiation therapy as hyperprolactinemia and hyperthyroidism were linked, respectively, to breastfeeding and pregnancy.
DISCUSSION
Various studies have proven that hormonal deficits following radiotherapy cause significant losses in terms of quality of life.[
Postactinic hypopituitarism has been reported in 37–77% of patients with anterior skull base lesions treated with conventional radiotherapy.[
Furthermore, radiotherapy in the anterior skull base has also proven to be burdened by several other side effects, including memory loss,[
In our sample, 18.75% of patients reported hormonal alterations.
The patients who developed hormonal deficits, as predicted, showed a significant reduction (P = 0.027) in terms of quality of life when compared to patients who did not develop hormonal deficits [
The mean radiation dose received by patients who reported hormonal alterations was 57.6 GyRBE, lower than the mean radiation dose in the sample of patients who did not show hormonal alterations (60.6 GyRBE). Analyzing the collected data, evidence shows that there is no statistically significant correlation (P = 0.34) between the dose of administered radiation and incidence of postactinic hypopituitarism. The main reason for this result is that different doses of particle therapy are standardized within protocols specifically designed to avoid iatrogenic damage of this type [
The losses in terms of quality of life and post-PT performance status were not statistically significant (P = 0.066) in the patient sample, but there was a trend toward worsening of overall health status (according to KPS) in patients with postactinic hypopituitarism when compared to patients with no postactinic hormonal alterations.
By comparing the data collected in this study with the meta-analysis performed by Appelman-Dijkstra et al.,[
We also compared the data collected in this study with other studies carried out in the past 10 years. The two studies that we mainly considered were: Pituitary dysfunction following cranial radiotherapy for adult-onset nonpituitary brain tumors by Kyriakakis et al.,[
The results showed by both studies strengthen our argument. Specifically, the first publication shows that a significant number of patients developed hypopituitarism following conventional radiotherapy (88.8%), while the second shows an increase in the incidence of hypopituitarism in time (from 47% 2 years after treatment to 89% 15 years after treatment). These findings further stress the importance of long-term follow-up visits in patients who have undergone some radiotherapy targeted to the sellar region.
Our study, however, does show some limitations. First of all, this is a retrospective analysis.
Moreover, the sample size is small and inhomogeneous in terms of tumor histology and previous treatment.
Given the different anatomy and radiosensitivity patterns in every patient, it is impossible to assess the exact dose of radiation received by the pituitary gland in a standard fashion (although the isodoses were collected for each patient). Furthermore, even though all patients were adults, radiosensitivity may vary with age. Local invasion, previous surgery, and comorbidities may also play a role, especially in the microvascular pattern of both portal and arterial pituitary vasculature.
The study is also limited by the fact that blood samples and MRIs were carried out in different laboratories and centers.
CONCLUSION
Particle therapy appears to be burdened by a lower incidence of side effects when compared to conventional radiotherapy, especially with regard to alterations in pituitary function.
For this reason, particle therapy could represent a new frontier in the treatment of tumors involving the sellar region. Further studies are needed to confirm a potential long-term advantage of PT over conventional radiotherapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ajmal A, McKean E, Sullivan S, Barkan A. Decreased quality of life (QoL) in hypopituitary patients: Involvement of glucocorticoid replacement and radiation therapy. Pituitary. 2018. 21: 624-30
2. Albani A, Ferraù F, Angileri FF, Esposito F, Granata F, Ferreri F. Multidisciplinary management of pituitary apoplexy. Int J Endocrinol. 2016. 2016: 7951536
3. Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, Neelis KJ, Biermasz NR, Romijn JA. Pituitary dysfunction in adult patients after cranial radiotherapy: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2011. 96: 2330-40
4. Appelman-Dijkstra NM, Malgo F, Neelis KJ, Coremans I, Biermasz NR, Pereira AM. Pituitary dysfunction in adult patients after cranial irradiation for head and nasopharyngeal tumours. Radiother Oncol. 2014. 113: 102-7
5. Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010. 76: S50-7
6. Biermasz NR, van Dulken H, Roelfsema F. Long-term follow-up results of postoperative radiotherapy in 36 patients with acromegaly. J Clin Endocrinol Metab. 2000. 85: 2476-82
7. Cancer Therapy Evaluation Progr, editors. Common Terminology Criteria for Adverse Events, Version 5.0. Maryland: DCTD, NCI, NIH, DHHS; 2017. p.
8. Crespo I, Santos A, Webb SM. Quality of life in patients with hypopituitarism. Curr Opin Endocrinol Diabetes Obes. 2015. 22: 306-12
9. Darzy KH, Shalet SM. Hypopituitarism following radiotherapy. Pituitary. 2009. 12: 40-50
10. De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AW, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Prim. 2019. 5: 13
11. Hansen JP. Can’t miss: Conquer any number task by making important statistics simple. Part 6. Tests of statistical significance (Z test statistic, rejecting the null hypothesis, P value), T test, Z test for proportions, statistical significance versus meaningful difference. J Healthc Qual. 2004. 26: 43-53
12. Kyriakakis N, Lynch J, Orme SM, Gerrard G, Hatfield P, Loughrey C. Pituitary dysfunction following cranial radiotherapy for adult-onset nonpituitary brain tumours. Clin Endocrinol (Oxf). 2016. 84: 372-9
13. Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010. 76: S28-35
14. Mishra P, Singh U, Pandey CM, Mishra P, Pandey G. Application of student’s T-test, analysis of variance, and covariance. Ann Card Anaesth. 2019. 22: 407-11
15. Orecchia R, Vitolo V, Fiore MR, Fossati P, Iannalfi A, Vischioni B. Proton beam radiotherapy: Report of the first ten patients treated at the “Centro Nazionale di Adroterapia Oncologica (CNAO)” for skull base and spine tumours. Radiol Med. 2014. 119: 277-82
16. Pagella F, Ugolini S, Zoia C, Matti E, Carena P, Lizzio R. Clivus pathologies from diagnosis to surgical multidisciplinary treatment. Review of the literature. Acta Otorhinolaryngol Ital. 2021. 41: S42-50
17. Pai HH, Thornton A, Katznelson L, Finkelstein DM, Adams JA, Fullerton BC. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: Demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001. 49: 1079-92
18. Scoccianti S, Detti B, Gadda D, Greto D, Furfaro I, Meacci F. Organs at risk in the brain and their dose-constraints in adults and in children: A radiation oncologist’s guide for delineation in everyday practice. Radiother Oncol. 2015. 114: 230-8
19. Skiba-Tatarska M, Kusa-Podkańska M, Surtel A, WysokińskaMiszczuk J. The side-effects of head and neck tumors radiotherapy. Pol Merkur Lekarski. 2016. 41: 47-9
20. Smith MM, Strottmann JM. Imaging of the optic nerve and visual pathways. Semin Ultrasound CT MR. 2001. 22: 473-87
21. Solari D, Zenga F, Angileri FF, Barbanera A, Berlucchi S, Bernucci C. A survey on pituitary surgery in Italy. World Neurosurg. 2019. 123: e440-9
22. Taku N, Gurnell M, Burnet N, Jena R. Time dependence of radiation-induced hypothalamic-pituitary axis dysfunction in adults treated for non-pituitary, intracranial neoplasms. Clin Oncol (R Coll Radiol). 2017. 29: 34-41
23. Tooze A, Gittoes NJ, Jones CA, Toogood AA. Neurocognitive consequences of surgery and radiotherapy for tumours of the pituitary. Clin Endocrinol (Oxf). 2009. 70: 503-11
24. Urie MM, Sisterson JM, Koehler AM, Goitein M, Zoesman J. Proton beam penumbra: Effects of separation between patient and beam modifying devices. Med Phys. 1986. 13: 734-41
25. Wilson RR. Radiological use of fast protons. Radiology. 1946. 47: 487-91
26. Zoia C, Lombardi F, Custodi VM, Lovati E, Lucotti P, Iannalfi A. Evaluation of the early endocrinological sequelae after hadron therapy on anterior skull base lesions in the adult population. Minerva Endocrinol. 2020. 45: 162-4
27. Zoli M, Guaraldi F, Zoia C, La Corte E, Asioli S, Bongetta D. Management of sellar and parasellar tumors becoming symptomatic during pregnancy: A practical algorithm based on multi-center experience and systematic literature review. Pituitary. 2021. 24: 269-83