- Department of Neurosurgery, Faculty of Medicine and Pharmacy, Mohammed V University of Rabat, Rabat, Morocco,
- Clinical Investigation Center (CIC), 1415, INSERM, Teaching Hospital of Tours, Tours, France.
Correspondence Address:
Yao Christian Hugues Dokponou, Department of Neurosurgery, Faculty of Medicine and Pharmacy, Mohammed V University of Rabat, Rabat, Morocco.
DOI:10.25259/SNI_592_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mahjouba Boutarbouch1, Yao Christian Hugues Dokponou1, Nourou Dine Adeniran Bankole1,2, Abdessamad El Ouahabi1, Abdeslam El Khamlichi1. Evaluation of unruptured aneurysm scoring systems and ratios in subarachnoid hemorrhage patients with multiple intracranial aneurysms. 20-Oct-2023;14:372
How to cite this URL: Mahjouba Boutarbouch1, Yao Christian Hugues Dokponou1, Nourou Dine Adeniran Bankole1,2, Abdessamad El Ouahabi1, Abdeslam El Khamlichi1. Evaluation of unruptured aneurysm scoring systems and ratios in subarachnoid hemorrhage patients with multiple intracranial aneurysms. 20-Oct-2023;14:372. Available from: https://surgicalneurologyint.com/surgicalint-articles/12602/
Abstract
Background: This study aims to appraise aneurysm scores and ratios’ ability to discriminate between ruptured aneurysms and unruptured intracranial aneurysms (UIAs) in subarachnoid hemorrhage (SAH) patients harboring multiple intracranial aneurysms (MICAs). We, then, investigate the most frequent risk factors associated with MICAs.
Methods: We retrospectively applied unruptured intracranial aneurysm treatment score (UIATS) and population hypertension age size of aneurysm earlier SAH from another aneurysm site of aneurysm (PHASES) score, aspect, and dome-to-neck ratio to the 59 consecutive spontaneous SAH patients with MICAs admitted between January 2000 and December 2015 to the Department of Neurosurgery of the University Hospital Center “Hôpital des Spécialités” of Rabat (Morocco). Patients with at least two intracranial aneurysms (IAs) confirmed on angiography were included in the study.
Results: Fifty-nine patients were harboring 128 IAs. The most frequent patient-level risk factors were arterial hypertension (AHT) 30.5 % (n = 18) and smoking status 22.0 % (n = 13). A PHASES score recommended treatment in 52 of 60 ruptured aneurysms and in six of 68 UIAs with a sensitivity of 31.67% and a specificity of 76.47%. UIATS recommended treatment in 26 of 62 ruptured aneurysms and in 35 of 55 UIAs with a sensitivity of 41.9% and a specificity of 63.6%. Aspect ratio recommended treatment in 60 of 60 ruptured aneurysms and in 63 of 68 UIAs with a sensitivity of 100% and a specificity of 88.24%. Dome-to-neck ratio recommended treatment in 45 of 60 ruptured aneurysms and in 48 of 68 UIAs with a sensitivity of 80% and a specificity of 63.24%. The aspect ratio (area under the curve [AUC] = 0.953) AUC > 0.8 has a higher discriminatory power between ruptured aneurysms and UIAs.
Conclusion: AHT and smoking status were the most common risk factors for intracranial multiple aneurysms and the aspect ratio and PHASES score were the most powerful discrimination tools between ruptured aneurysms and the UIAs.
Keywords: Multiple intracranial aneurysms, Ruptured aneurysm, Scores and ratio, Unruptured aneurysm
INTRODUCTION
Multiple intracranial aneurysm (MICA) counts approximately 30% of patients with intracranial aneurysms (IAs).[
In the case of MICA, identifying the ruptured IA is increasingly important in such an era; here, patients are mostly treated by endovascular means, it is difficult to confirm visually the source of hemorrhage, and aneurysms are usually treated individually.[
However, Orning et al.[
Rajabzadeh-Oghaz et al.[
However, MICA management is still challenging nowadays whether revealed by SAH or not, and the indication of adequate treatment might be case by case after a meeting between neurosurgeons or hybrid neurosurgeons and interventional neuroradiologist team.
This study aims to appraise aneurysm scores and ratios’ ability to discriminate between ruptured aneurysms and unruptured intracranial aneurysms (UIAs) in SAH patients harboring MICAs. We, then, investigate the most frequent risk factors associated with MICAs.
MATERIALS AND METHODS
Ethics statement
The data collected during the study have been stored in a computer file in conformity with the Moroccan Data Protection Law, Decree n° 2-09-165 of May 21, 2009. The study consent was waived because this was a retrospective study with anonymized data collection.
Study population
This is a cohort retrospective single institutional review of patients diagnosed with spontaneous SAH, from January 2000 to December 2015 in the Department of Neurosurgery of the University Hospital Center “Hôpital des Spécialités” of Rabat (Morocco). Patients diagnosed with at least two IAs confirmed on angiography were included in this study. The ruptured aneurysms were pinpointed among SAH patients secondary to the aneurysmal rupture confirmed on the cerebral arteriography. We excluded all patients with single IAs, incomplete medical records, and all others who do not have a cerebral angiographic result in their record.
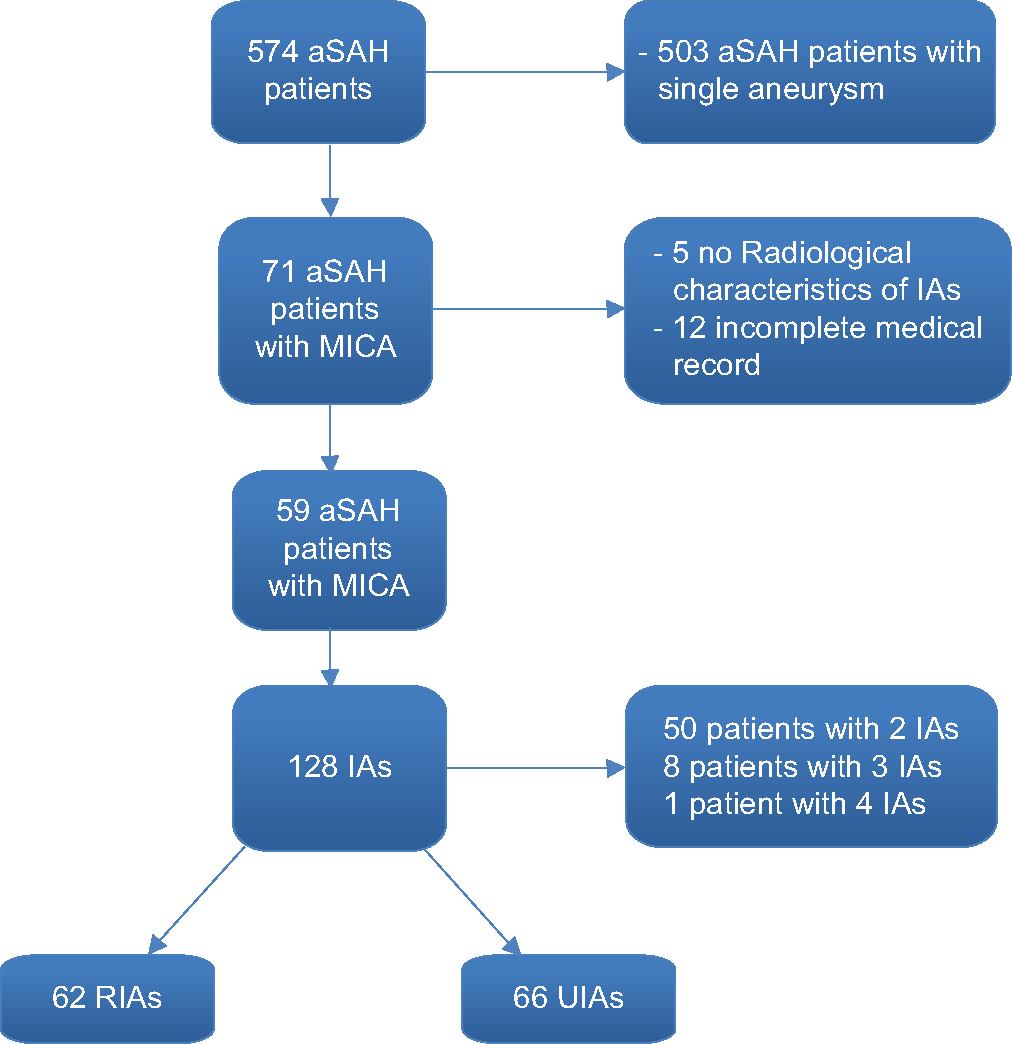
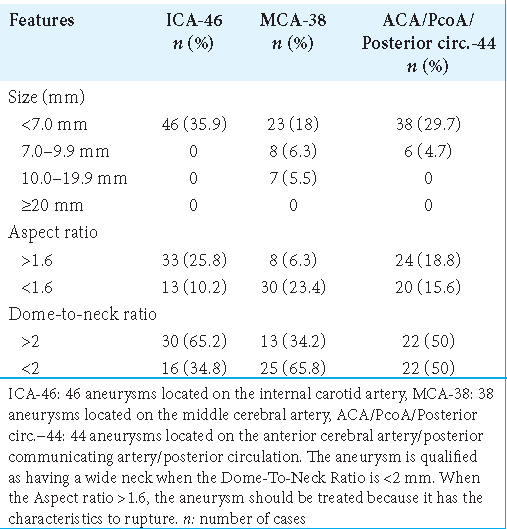
A total of 574 patients were admitted between January 2000 and December 2015 to the Department of Neurosurgery of the University Hospital Center “Hôpital des Spécialités” of Rabat (Morocco) with the diagnosis of spontaneous SAH. Of these, 59 (10.3%) patients were diagnosed with multiple aneurysms, harboring 128 aneurysms. Fifty (84.7%) patients were diagnosed with two, 8 (13.6%) patients with three, and only 1 (1.7%) patient with four aneurysms [
Data acquisition
Patient-level sociodemographic data (age and gender), medical history, risk factors, WFNS and Fisher grading, number of aneurysms per patient, clinical state, date of management, surgical, endovascular, and conservative treatment, complications, outcome, and follow-up as well as aneurysm level data such as size, neck, width, height, dome-to-neck ratio, topography of aneurysm, aspect ratio, ruptured aneurysm, and unruptured aneurysm were collected and tabulated in an Excel spreadsheet. IASCORE[
Statistical analysis
Data analysis was performed using JAMOVI version 3.2.8. Differences in dome-to-neck ratio, aspect ratio, PHASES score, and UIATS between ruptured IAs and UIAs were determined by fitting linear mixed models with a patient-specific random intercept. The within-patient and between-patient standard deviations were estimated based on the fitted models. Regression coefficients were rescaled according to within-patient standard deviations to compare the ability of both ratios and scores to discriminate between ruptured aneurysms and UIAs in the same patient. In addition, we calculated areas under the receiver operating characteristic (ROC) curves for both ratios and scores for each patient’s aneurysms and compared them statistically using the Likelihood ratio. Conditional logistic regression models were fitted to aneurysm type (ruptured aneurysm vs. UIA) to investigate the ability of the different aneurysm-specific characteristics of ratios and scores to discriminate between ruptured aneurysms and UIAs of the same patient. The likelihood ratio Chi-squared test and the Akaike information criterion were used to compare the models, and revised scores were generated based on the selected model coefficients. We, then, broke through the one-to-many matching of patients and compared the pool of 62 ruptured aneurysms to 66 UIAs to calculate sensitivity and specificity by applying the described score cutoffs. We similarly generated ROC curves to analyze the ability of each ratio and score to marginally discriminate between ruptured IAs and UIAs. All P-values are two-tailed. P-values below 0.05 were considered statistically significant.
RESULTS
Patient cohort and aneurysms characteristics
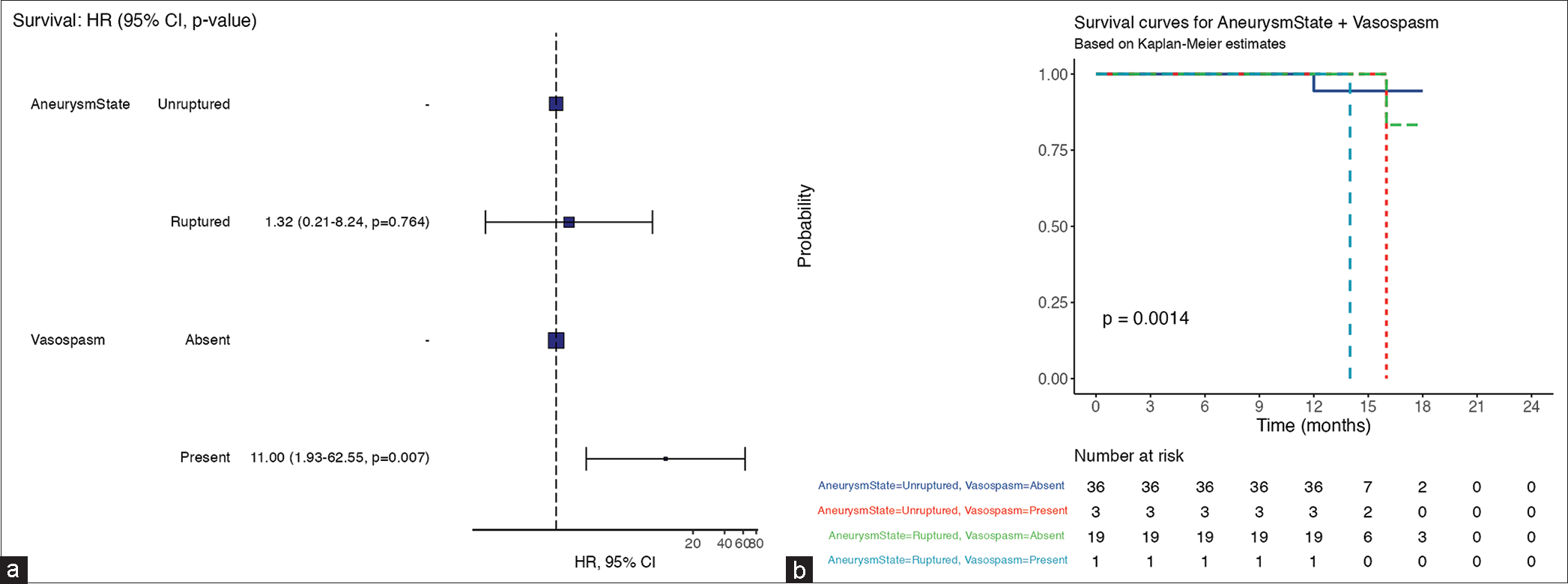
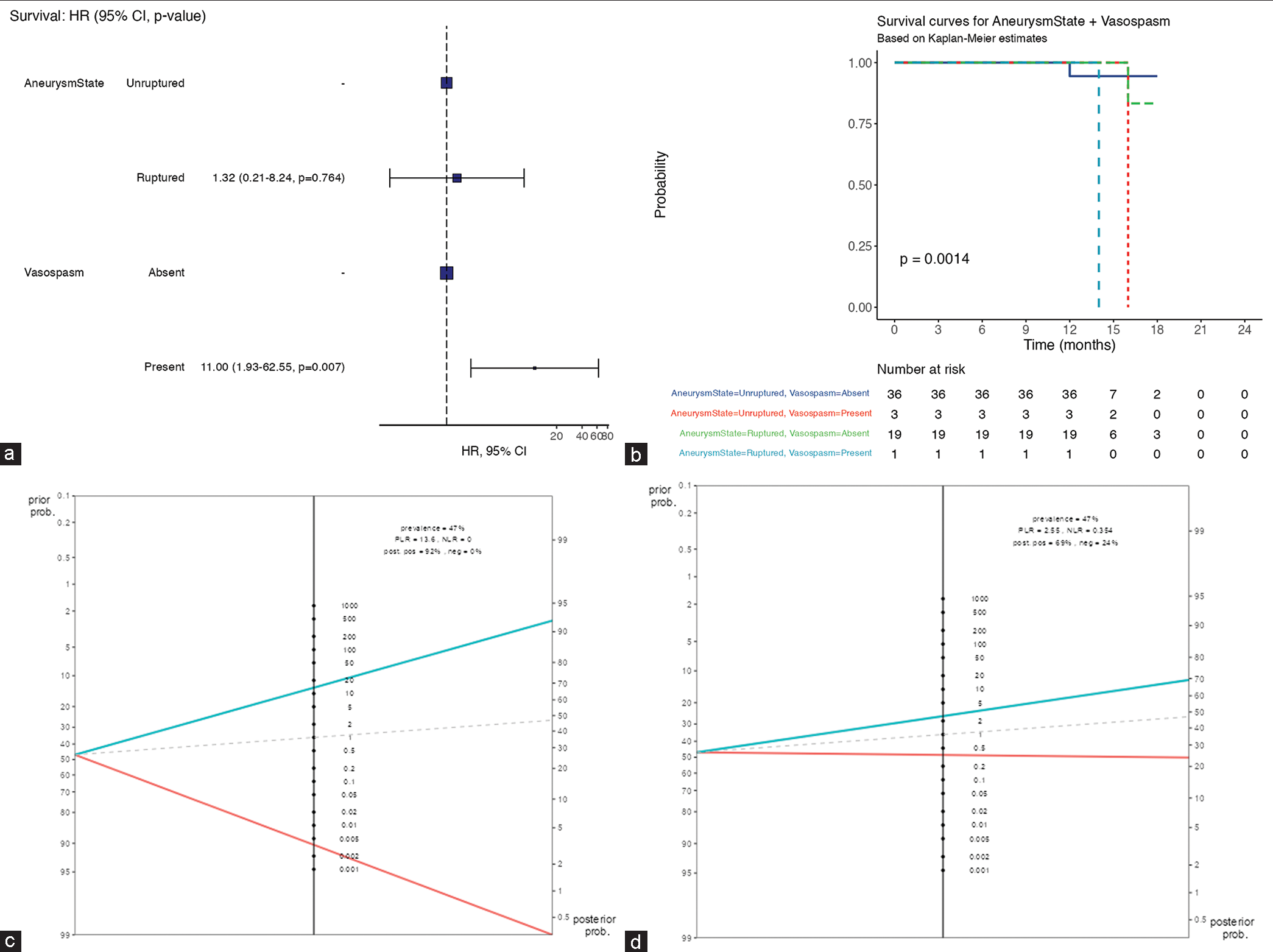
The most frequent patient-level risk factors were arterial hypertension (AHT) 30.5% (n = 18), followed by current smoking 22.0% (n = 13). Diabetes and combined arterial hypertension plus diabetes count for 11.9% (n = 7) for each. Forty-one (69.5%) patients presented with WFNS Grade I whereas 24 (40.7%) were Grade IV of Fisher. Interestingly, the multivariable survival analysis of
Figure 2:
(a) Multivariable survival analysis showing statistically significant high mortality rate (heart ratio = 11, P < 0.05) among subarachnoid hemorrhage patients complicated with vasospasm. (b) In a short-term follow-up, the overall patient with vasospasm is at risk of dying within 14 months after aneurysms ruptured. HR: Hazard ratio, CI: Confident interval
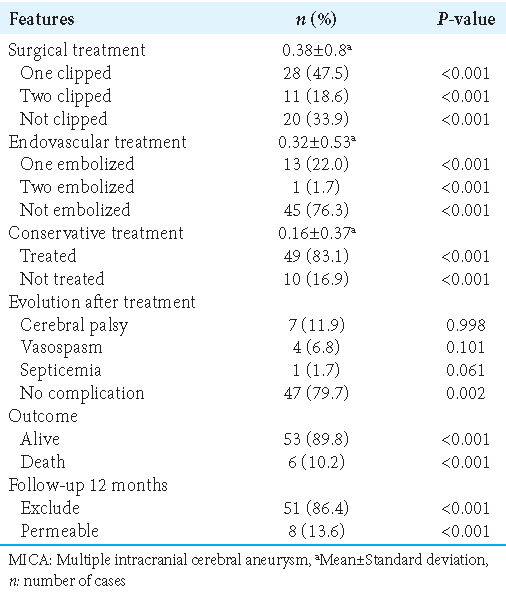
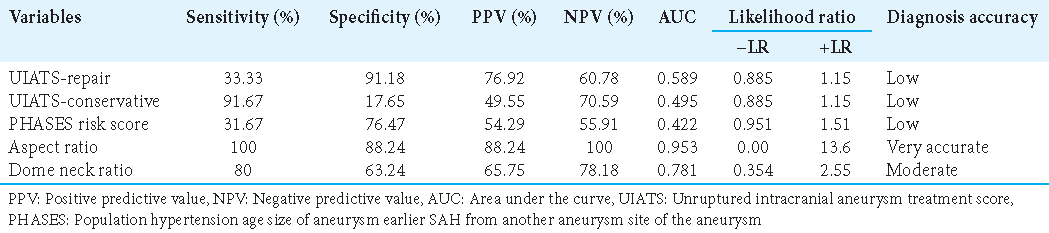
The UIATS recommends aneurysm treatment in 71 cases (55.5 %), conservative management in 46 cases (35.9%), and inconclusive in 11 cases (8.6 %). For the 66 UIAs, UIATS recommended aneurysm treatment in 35 (27.3%), conservative management in 25 (19.5%), and inconclusive in 6 (4.7%) cases. Among the 62 ruptured IAs, the mean estimated rupture rate over 5 years according to PHASES score was 1.25% ± 1.3. In 58 (45.3%) cases, the PHASES rupture rate over 5 years was <5% and >5% for only 4(3.1%) cases. A detailed overview of the UIATS and the distribution of the PHASES score for ruptured aneurysms and UIAs are shown in
UIATS and PHASES Score discrimination versus aspect and dome-to-neck ratio
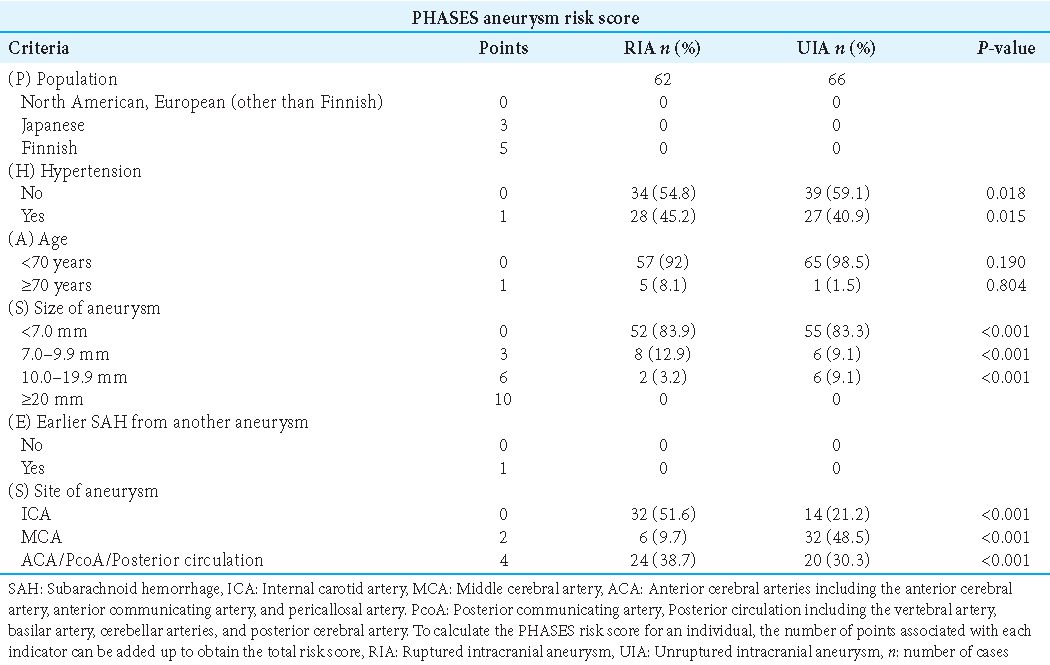
The sensitivity and specificity of these variables were applied to the ruptured aneurysms and the UIAs, assuming that for ruptured aneurysms, the decision for treatment, and UIAs for conservative management would be correct. A PHASES score of ≥6 points was considered as a recommendation for treatment, a score of ≥1.5<6 (1.5; 6) indicated a low likelihood of aneurysm rupture. With these settings, the PHASES score recommended treatment in 52 of 60 ruptured aneurysms and 6 of 68 UIAs, resulting in a sensitivity of 31.67% and a specificity of 76.47%. UIATS recommended treatment when the number of points in favor of repair is ≥17 and the number of points in favor of conservative management is ≤10. With these settings, the UIATS recommended treatment in 26 of 62 ruptured aneurysms and 35 of 55 UIAs with a sensitivity of 41.9% and a specificity of 63.6%. Treatment is recommended when the aspect ratio is >1.6. With these settings, the aspect ratio recommended treatment in 60 of 60 ruptured aneurysms and 63 of 68 UIAs with a sensitivity of 100% and a specificity of 88.24%. Treatment is recommended when the dome-to-neck ratio is <1.8. With these settings, the dome-to-neck ratio recommended treatment in 45 of 60 ruptured aneurysms and 48 of 68 UIAs with a sensitivity of 80% and a specificity of 63.24% [
Figure 3:
Fagan nomogram (a) the UIATS recommendation for aneurysm repair/conservative treatment was from 47% to 49% of cases when rupture is predicted (positive likelihood ratio 1.15; negative likelihood ratio 0.885). (b) The prevalence of PHASES risk score to predict aneurysm rupture was from 47% to 57% when the rupture happened as predicted (positive likelihood ratio 1.51; negative likelihood ratio 0.951). (c) The prevalence of aspect ratio to predict aneurysm rupture was 47% at the pretest and 92% post-test when the rupture happened as predicted (positive likelihood ratio 13.6; negative likelihood ratio 0.00). (d) The prevalence of dome-to-neck ratio to predict aneurysm rupture was 47% at the pretest and 69% post-test when the rupture happened as predicted (positive likelihood ratio 2.55; negative likelihood ratio 0.354). UIATS: Unruptured intracranial aneurysm treatment score, PHASES: Population hypertension age size of aneurysm earlier SAH from another aneurysm site of aneurysm. HR: Hazard ratio, CI: Confident interval, PLR: Positive likelihood ratio, NLR: Negative likelihood ratio
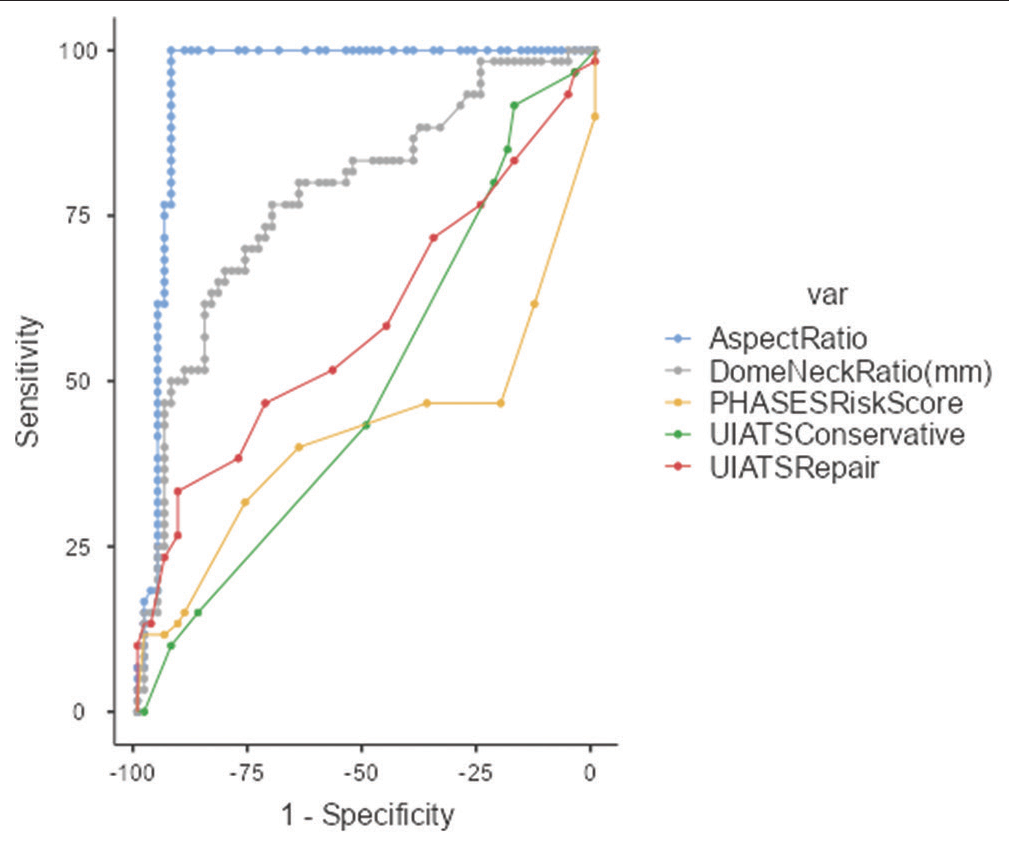
To investigate the scores’ ability to discriminate between ruptured aneurysms and UIAs, a ROC curve analysis was performed; the results are shown in [
Figure 4:
The ROC curve of UIATS recommendation for repair/ conservative treatment and PHASES risk score is very far from the perfect discrimination point. The dome-to-neck ratio is closer to the previous curves but it’s AUC = 0.78 < 0.8. Meanwhile, the aspect ratio ROC curve passes on the perfect discrimination point with an AUC = 0.95 > 0.8. ROC: Receiver operating characteristic, UIATS: Unruptured intracranial aneurysm treatment score, PHASES: Population hypertension age size of aneurysm earlier SAH from another aneurysm site of aneurysm, AUC: Area under the curve.
DISCUSSION
Key findings
The most frequent patient-level risk factors for MICAs were AHT 30.5% (n = 18) and smoking status 22.0% (n = 13).
Treatment is recommended when the aspect ratio is >1.6. The aspect ratio (AUC = 0.953) AUC >0.8 is the most powerful discrimination tool between ruptured aneurysms and the UIAs.
Implications
Hadjiathanasiou et al.[
Vasospasm is a complication with a higher risk of mortality (HR = 11, P < 0.05) within 14 months in our cohort of ruptured aneurysms with MICA patients. On the other hand, Baumann et al.[
Previously, Mocco et al.,[
Limitations
One limitation of this study is the relatively small sample size. In addition, this is a monocentric retrospective study. There were only three populations “North American, European (other than Finnish), Japanese, and Finnish” that were considered for the PHASES Score establishment. The lack of many more populations such as the Sub-Saharan Africa, the Maghrebin, and the Arabic should be considered in the interpretation of the results from this study that, nonetheless, is the first step toward attribution of “points” to each of those populations as criteria after knowing the risk rate of IAs rupture in each population. This will most probably give the most accurate sensitivity and specificity of each variable, either scores or ratio in predicting the likelihood of the rupture of IA. A randomized control trial is needed to fix this scarcity of scientific data.
CONCLUSION
Our findings underlined clearly that the aspect ratio and the PHASES score are the most powerful discrimination tools between ruptured aneurysms and the UIAs in patients harboring MICA. This might be a helpful tool to predict the risk of rebleeding as well as the risk of vasospasm which is correlated with a high mortality rate. Future studies like a randomized control trial should be able to allow adding some other population groups to the PHASES Score items.
Submission statement
This manuscript is original and has not been submitted elsewhere.
Disclosures
The authors have nothing to disclose.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Asgharzadeh H, Borazjani I. Effects of Reynolds and Womersley numbers on the hemodynamics of intracranial aneurysms. Comput Math Methods Med. 2016. 2016: 7412926
2. Baumann F, Khan N, Yonekawa Y, editors. Patient and aneurysm characteristics in multiple intracranial aneurysms. Changing aspects in stroke surgery: Aneurysms, dissections, moyamoya angiopathy and EC-IC bypass. Germany: Springer; 2008. p. 19-28
3. Bhogal P, AlMatter M, Hellstern V, Ganslandt O, Bäzner H, Henkes H. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Surg Neurol Int. 2018. 9: 1
4. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008. 63: 185-96
5. Dharshini P, Raghunath G, Gurusamy K, Begum Z, Dhamodaran S, Karunakaran B. Morphometric study of the intracranial segment of the vertebral artery. Cureus. 2022. 14: e22137
6. Feghali J, Gami A, Xu R, Jackson CM, Tamargo RJ, McDougall CG. Application of unruptured aneurysm scoring systems to a cohort of ruptured aneurysms: Are we underestimating rupture risk?. Neurosurg Rev. 2021. 44: 3487-98
7. Filipce V, Caparoski A. The effects of vasospasm and re-bleeding on the outcome of patients with subarachnoid hemorrhage from ruptured intracranial aneurysm. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015. 36: 77-82
8. Hadjiathanasiou A, Schuss P, Brandecker S, Welchowski T, Schmid M, Vatter H. Multiple aneurysms in subarachnoid hemorrhage-identification of the ruptured aneurysm, when the bleeding pattern is not self-explanatory-development of a novel prediction score. BMC Neurol. 2020. 20: 70
9. Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000. 31: 392-7
10. Kashiwazaki D, Kuroda S. Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke. 2013. 44: 2169-73
11. Kozyrev DA, Jahromi BR, Thiarawat P, Choque-Velasquez J, Ludtka C, Goehre F. Three distal anterior cerebral artery aneurysms in the same branch associated with five additional intracranial aneurysms. Surg Neurol Int. 2017. 8: 62
12. Mocco J, Brown RD, Torner JC, Capuano AW, Fargen KM, Raghavan ML. Aneurysm morphology and prediction of rupture: An international study of unruptured intracranial aneurysms analysis. Neurosurgery. 2018. 82: 491-6
13. Murray CJ, editors. The global burden of disease: Summary; a comprehensive Assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Geneva: World Health Organization; 1996. p.
14. Nehls DG, Flom RA, Carter LP, Spetzler RF. Multiple intracranial aneurysms: Determining the site of rupture. J Neurosurg. 1985. 63: 342-8
15. Neulen A, Pantel T, König J, Brockmann MA, Ringel F, Kantelhardt SR. Comparison of unruptured intracranial aneurysm treatment score and PHASES score in subarachnoid hemorrhage patients with multiple intracranial aneurysms. Front Neurol. 2021. 12: 616497
16. Orning JL, Shakur SF, Alaraj A, Behbahani M, Charbel FT, Aletich VA. Accuracy in identifying the source of subarachnoid hemorrhage in the setting of multiple intracranial aneurysms. Neurosurgery. 2018. 83: 62-8
17. Raghavan ML, Ma B, Harbaugh RE. Quantified aneurysm shape and rupture risk. J Neurosurg. 2005. 102: 355-62
18. Rahman M, Smietana J, Hauck E, Hoh B, Hopkins N, Siddiqui A. Size ratio correlates with intracranial aneurysm rupture status: A prospective study. Stroke. 2010. 41: 916-20
19. Rajabzadeh-Oghaz H, Wang J, Varble N, Sugiyama SI, Shimizu A, Jing L. Novel models for identification of the ruptured aneurysm in patients with subarachnoid hemorrhage with multiple aneurysms. AJNR Am J Neuroradiol. 2019. 40: 1939-46
20. Rajabzadeh-Oghaz H, Varble N, Shallwani H, Tutino VM, Mowla A, Shakir HJ. Computer-assisted three-dimensional morphology evaluation of intracranial aneurysms. World Neurosurg. 2018. 119: e541-50
21. Sato H, Kamide T, Kikkawa Y, Kimura T, Kuribara S, Yanagawa T. Clinical characteristics of ruptured intracranial aneurysm in patients with multiple intracranial aneurysms. World Neurosurg. 2021. 149: e935-41
22. Sharma P, Mehrotra A, Das KK, Bhaisora KS, Sardhara J, Godbole CA. Factors predicting poor outcome in a surgically managed series of multiple intracranial aneurysms. World Neurosurg. 2016. 90: 29-37
23. Sharma S, Krishna H, Dixit SG, Nayyar AK, Khera P, Ghatak S. Systematic review of morphometric analysis of anterior cerebral artery (ACA) emphasizing on its clinical implications. Cureus. 2023. 15: e37744
24. Tsivgoulis G, Sharma VK, Hoover SL, Lao AY, Ardelt AA, Malkoff MD. Applications and advantages of power motion-mode Doppler in acute posterior circulation cerebral ischemia. Stroke. 2008. 39: 1197-204
25. Tykocki T, Nauman P, Enko AD. Morphometric predictors of posterior circulation aneurysms risk rupture. Neurol Res. 2014. 36: 733-8
26. Xiang J, Antiga L, Varble N, Snyder KV, Levy EI, Siddiqui AH. AView: An image-based clinical computational tool for intracranial aneurysm flow visualization and clinical management. Ann Biomed Eng. 2016. 44: 1085-96
27. Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. 2011. 42: 144-52