- Department of Neurosurgery, Saiseikai Shiga Hospital, Imperial Gift Foundation Inc., Ritto, Shiga, Japan
- Department of Laboratory Medicine, Saiseikai Shiga Hospital, Imperial Gift Foundation Inc., Ritto, Shiga, Japan
Correspondence Address:
Shigeomi Yokoya, Department of Neurosurgery, Saiseikai Shiga Hospital, Imperial Gift Foundation Inc., Ritto, Shiga, Japan.
DOI:10.25259/SNI_684_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shigeomi Yokoya1, Akinori Kurimoto2. Exclusion of echo-lucent plaque using superb micro-vascular imaging: A case report. 11-Oct-2024;15:373
How to cite this URL: Shigeomi Yokoya1, Akinori Kurimoto2. Exclusion of echo-lucent plaque using superb micro-vascular imaging: A case report. 11-Oct-2024;15:373. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13142
Abstract
Background: In the carotid bulb (CB), the vascular morphology can cause a decrease in blood flow velocity near the vessel wall. In addition, the CB is a common site for plaque formation. Particularly, echo-lucent plaques (ELPs) are known to pose a risk for cerebral embolism, requiring careful attention. In carotid ultrasonography (CU), ELPs may be difficult to distinguish from blood flow within the vessel using only B-mode imaging; thus, the use of color Doppler imaging (CDI) is recommended. However, when blood flow is extremely slow, even CDI may fail to differentiate between ELPs and the flow. We encountered a case where superb micro-vascular imaging (SMI) successfully detected extremely low-velocity blood flow, thereby excluding the presence of an ELP that CDI could not discern.
Case Description: A 64-year-old male with a history of smoking, hyperlipidemia, and percutaneous coronary intervention for myocardial infarction presented for an atherosclerosis screening. CU with CDI indicated a lesion showing a flow void near the wall of the CB, raising suspicions of significant blood flow stasis or the presence of an ELP or thrombus. He had no neurological findings or carotid bruits. A head magnetic resonance imaging revealed no findings suggestive of cerebral embolization. Using SMI during additional CU, we detected extremely low-velocity blood flow near the wall of the CB, allowing us to exclude an ELP.
Conclusion: When a flow void is observed with CDI in CU, and it is difficult to differentiate between an ELP and extremely low-velocity blood flow, the application of SMI can sometimes detect the extremely low-velocity blood flow. This approach may help avoid invasive examinations such as CU with contrast agents or cerebral angiography.
Keywords: Carotid ultrasonography (CU), Color Doppler imaging (CDI), Echo-lucent plaque (ELP), Extremely low-velocity blood flow, Superb micro-vascular imaging (SMI)
INTRODUCTION
In the carotid bulb (CB) of the carotid artery, blood flow velocity decreases near the vessel wall due to the vessel’s morphology. The CB is a common site for plaque formation. Pathologically, echo-lucent plaques (ELPs), observed as low-echoic areas compared to the intima-media complex in carotid ultrasonography (CU), contain a large lipid core covered with a thin fibrous cap and are considered a stroke risk factor. Thus, when performing CU, combining color Doppler imaging (CDI) with B-mode ultrasound imaging (BMI) is essential to avoid overlooking plaques, as ELPs are difficult to distinguish from blood flow.
Despite the utility of CDI, it has limitations in displaying slow flow velocities in the carotid artery. When the velocity range is set low to observe low-velocity blood flow, the movement of the tissue surrounding the carotid artery itself can be displayed as tissue motion artifact signals, making it difficult to observe the blood flow in the vessel, which presents a dilemma. Superb micro-vascular imaging (SMI) is a newer technique designed to overcome these limitations by analyzing tissue motion characteristics and reducing motion artifacts, allowing for the separation of extremely low-velocity blood flow from tissue motion. SMI has proven effective in evaluating neovascularization and complex ulcerative lesions within plaques,[
To the best of our knowledge, there have been no reports of SMI distinguishing extremely low-velocity blood flow in the CB from an ELP. We present a case where using SMI to visualize extremely low-velocity blood flow, which could not be visualized using CDI, allowed us to rule out the presence of an ELP.
CASE DESCRIPTION
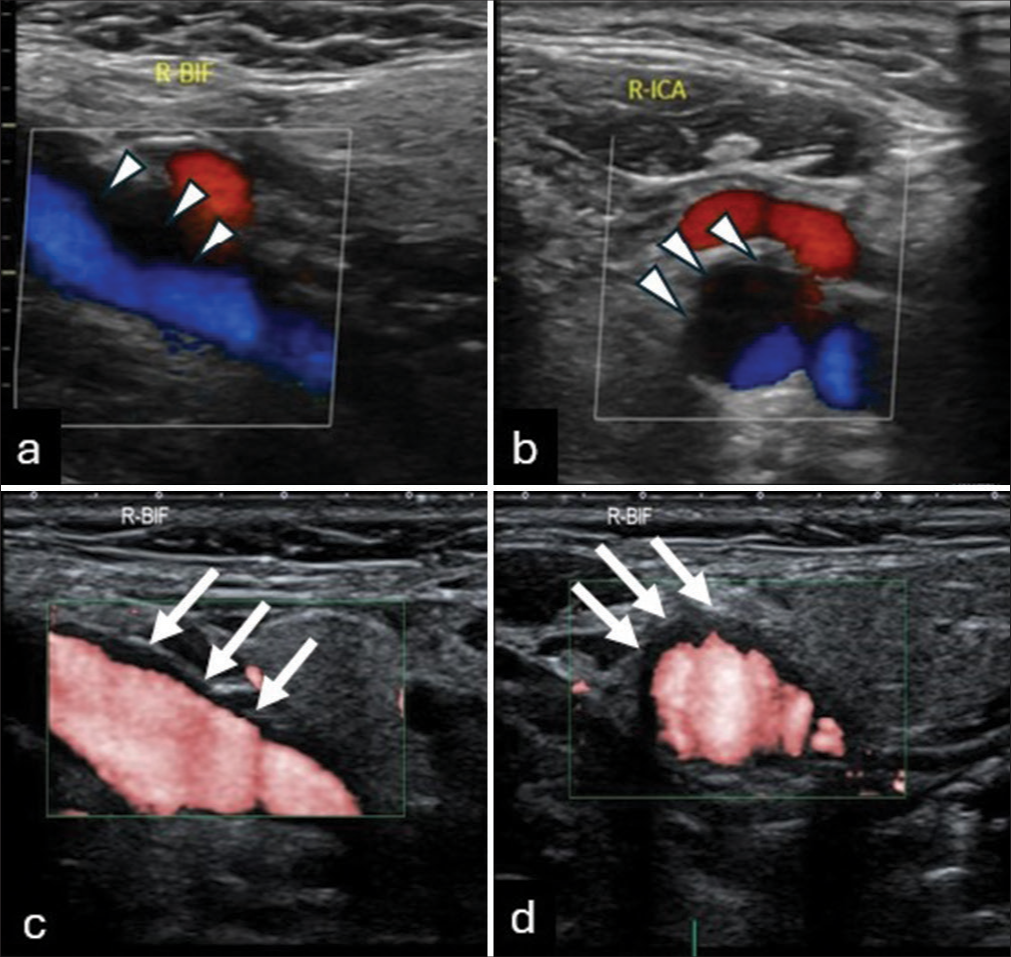
A 64-year-old man with hypertension, hyperlipidemia, and a history of myocardial infarction treated with percutaneous coronary intervention 15 years ago underwent CU for atherosclerosis evaluation. On ultrasound, BMI and CDI revealed an echo-lucent area with a flow void at his CB [
Figure 1:
(a and b) Color Doppler ultrasound images, in the major axis (a) and minor axis (b), showing an echo-lucent area (arrowheads) with a flow void signal near the wall of the carotid bulb (CB). (c and d) Superb micro-vascular imaging in the major axis (c) and minor axis (d) shows extremely low-velocity blood flow signals (white arrows) at the CB during systole.
DISCUSSION
In this case, a flow void was observed at the CB on CDI, initially raising suspicions of significant blood flow stasis or the presence of a low-echo thrombus or an ELP. Using SMI, we observed blood flow in the flow void area detected by CDI, concluding that the flow void pointed out by the CDI was due to extremely low-velocity blood flow.
This case offers several clinical insights. First, blood flow near the vessel wall at the CB can slow to the point where CDI fails to detect it. In the carotid artery, blood flow varies due to vessel morphology (diameter, stenosis, bends, and branches), pulsatile turbulence, blood viscosity, plasma protein concentration, red blood cell (RBC) concentration, RBC shape, RBC membrane elasticity, hematocrit, and body temperature. In addition, blood flow velocity is maximum at the central axis of the vessel, decreasing toward the periphery. These factors contribute to complex blood flow changes in the CB.[
CDI can adjust its range to detect flow velocity as low as 0.60 cm/s in ultrasound equipment. However, tissue around the human carotid artery moves at speeds of 4–5 cm/s (based on our measurements using pulsed Doppler imaging), making it difficult to distinguish extremely low-velocity blood flow from surrounding tissue motion, resulting in tissue motion artifact signals on the CDI.
Second, SMI is an effective method for detecting extremely low-velocity blood flow in the CB, overcoming CDI’s limitations by reducing tissue motion artifacts. Through this ingenuity, SMI effectively visualizes fine, extremely low-velocity blood flow with high sensitivity, resolution, and minimal artifacts.[
For detecting extremely low-velocity blood flow in the carotid artery, CEUS is an option. However, CEUS requires intravenous contrast administration; the microbubble contrast effect depends on blood flow velocity, and some hospitals may not be able to perform the examination.[
CONCLUSION
Distinguishing an ELP from extremely low-velocity blood flow using CDI is challenging, but SMI can provide accurate assessments, potentially reducing the need for invasive examinations.
Ethical approval
The research/study approved by the Institutional Review Board at Saiseikai Shiga Hospital, number 633, dated March 7, 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Hagiwara Y, Fukano T, Kaburagi M, Akasu Y, Shimizu T, Hidaka G. An interesting case suggesting a correction of intra-plaque neovascularization and plaque protrusion in carotid artery stenting. Neurosonology. 2021. 34: 148-52
2. Hoshino M, Shimizu T, Ogura H, Hagiwara Y, Takao N, Soga K. Intraplaque microvascular flow signal in superb microvascular imaging and magnetic resonance imaging carotid plaque imaging in patients with atheromatous carotid artery stenosis. J Stroke Cerebrovasc Dis. 2018. 27: 3529-34
3. Nagai H, editors. 2 Physiology of blood flow. Manual of neurosonology 2020. The Japan Academy of Neurosonology. Tokyo: Soubunsha Co.; 2020. p. 16-20
4. Nakagawa F, Nagai H, Urushimatsu S, Fujiwara Y, Kambara M, Yoshikane T. A Doppler flow analysis of the neovascular flow in carotid plaque using ultrasound micro-flow imaging. Shimane J Med Sci. 2020. 37: 133-40
5. Russell DA, Wijeyaratne SM, Gough MJ. Changes in carotid plaque echomorphology with time since a neurologic event. J Vasc Surg. 2007. 45: 367-72
6. Saito K, Nagatsuka K, Ishibashi-Ueda H, Watanabe A, Kannki H, Iihara K. Contrast-enhanced ultrasound for the evaluation of neovascularization in atherosclerotic carotid artery plaques. Stroke. 2014. 45: 3073-5
7. Song ZZ, Zhang YM. Contrast-enhanced ultrasound imaging of the vasa vasorum of carotid artery plaque. World J Radiol. 2015. 7: 131-3
8. Staub D, Partovi S, Schinkel AF, Coll B, Uthoff H, Aschwanden M. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. 2011. 258: 618-26
9. Sztajzel R, Momjian S, Momjian-Mayor I, Murith N, Djebaili K, Boissard G. Stratified gray-scale median analysis and color mapping of the carotid plaque: Correlation with endarterectomy specimen histology of 28 patients. Stroke. 2005. 36: 741-5
10. Zhang H, Du J, Wang H, Wang H, Jiang J, Zhao J. Comparison of diagnostic values of ultrasound micro-flow imaging and contrast-enhanced ultrasound for neovascularization in carotid plaques. Exp Ther Med. 2017. 14: 680-8