- Department of Neurosurgery, Institute of Biomedical Biosciences, Tokushima University Graduate School, Tokushima, Japan.
- Department of Clinical Neurosciences, Institute of Biomedical Biosciences, Tokushima University Graduate School, Tokushima, Japan.
Correspondence Address:
Shu Sogabe, Department of Neurosurgery, Institute of Biomedical Biosciences, Tokushima University Graduate School, Tokushima, Japan.
DOI:10.25259/SNI_608_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shu Sogabe1, Tatsuya Haboshi1, Izumi Yamaguchi1, Masaaki Korai1, Nobuaki Yamamoto2, Kenji Shimada1, Yasuhisa Kanematsu1, Yasushi Takagi1. Experience of using coronary perfusion balloon catheter for acute middle cerebral artery occlusion. 13-Oct-2023;14:365
How to cite this URL: Shu Sogabe1, Tatsuya Haboshi1, Izumi Yamaguchi1, Masaaki Korai1, Nobuaki Yamamoto2, Kenji Shimada1, Yasuhisa Kanematsu1, Yasushi Takagi1. Experience of using coronary perfusion balloon catheter for acute middle cerebral artery occlusion. 13-Oct-2023;14:365. Available from: https://surgicalneurologyint.com/surgicalint-articles/12592/
Abstract
Background: We present the case of an individual with acute occlusion of the middle cerebral artery caused by atherosclerosis. The patient underwent angioplasty using a coronary perfusion balloon, which resulted in a favorable clinical outcome.
Case Description: A 66-year-old male patient presented with an acute onset of right hemiplegia and dysarthria. Magnetic resonance imaging revealed an occlusion of the left middle cerebral artery, and alteplase was administered, followed by a mechanical thrombectomy and intracranial balloon catheter angioplasty. Due to restenosis, a coronary perfusion balloon catheter was used for a 15-minute angioplasty procedure while maintaining the perfusion. This treatment approach led to the recanalization of the artery and favorable clinical outcomes.
Conclusion: The coronary perfusion balloon may represent a viable therapeutic alternative for the management of refractory intracranial atherosclerotic large vessel occlusion.
Keywords: Angioplasty, Intracranial atherosclerotic disease, Perfusion balloon
INTRODUCTION
A meta-analysis of several randomized controlled trials[
However, restenosis and reocclusion are highly prevalent due to residual plaque in the vessel wall of patients with intracranial atherosclerotic disease (ICAD). An additional angioplasty is considered for the atherosclerotic lesions.
Perfusion balloons, which allow a prolonged balloon dilation while maintaining peripheral perfusion, can be used during angioplasty for coronary artery lesions.
In this case, the patient had an acute middle cerebral artery occlusion and underwent thrombus retrieval and angioplasty using a cerebrovascular balloon catheter. However, a restenosis was observed. Therefore, a prolonged angioplasty was performed using a coronary perfusion balloon to maintain recanalization, ultimately leading to favorable outcomes.
CASE PRESENTATION
Case
66-years-old male.
Chief complaint
A sudden onset of the right hemiplegia and dysarthria.
Medical history
Nothing significant.
Life history
Smoked 30 cigarettes/day for 45 years.
History
At 7:30 a.m. while driving, the patient suddenly experienced right hemiplegia and dysarthria and was rushed to our hospital.
Neurological findings
Right facial paralysis, dysarthria, right upper extremity paralysis, right upper and lower extremity paresthesia, and National Institutes of Health Stroke Scale (NIHSS) score 6/42.
Neuroradiological findings
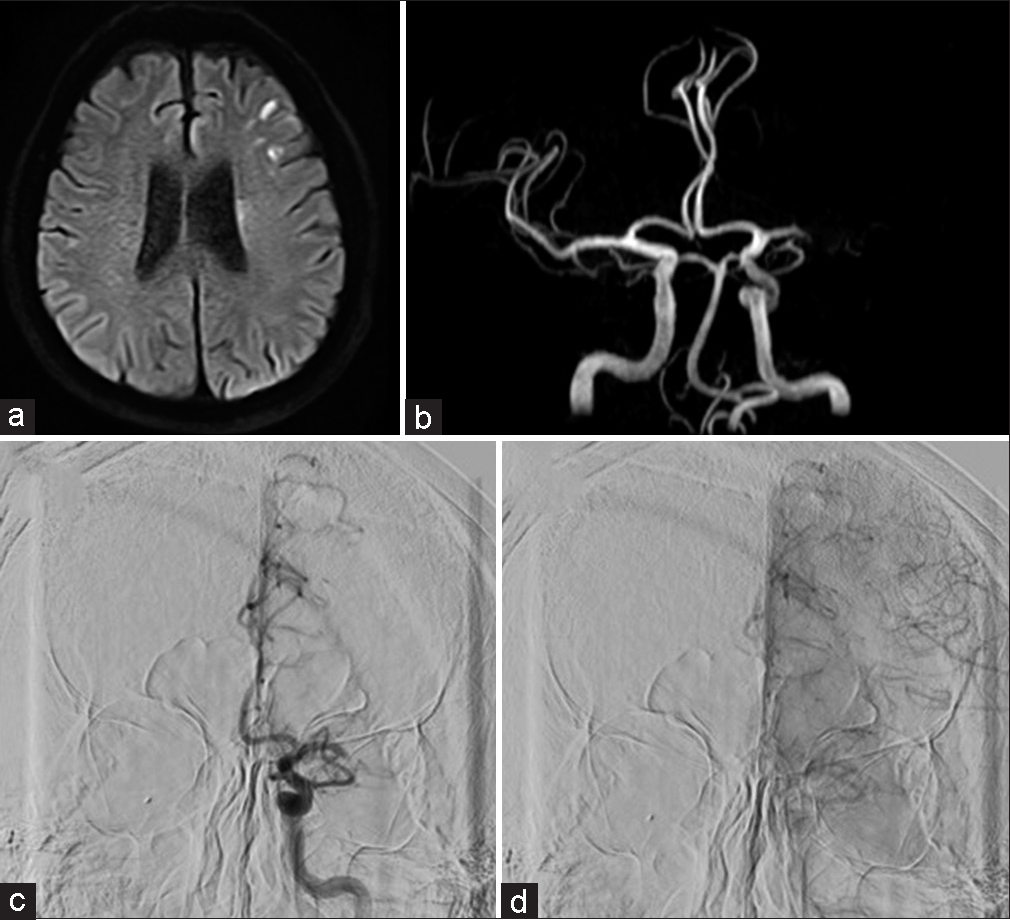
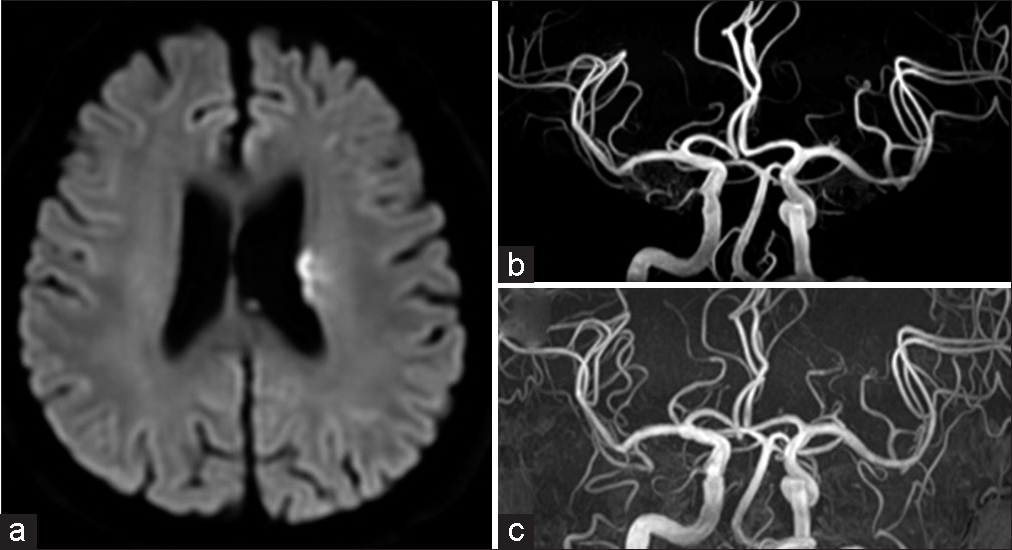
Magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) showed slightly high signal areas in the left putamen, corona radiata, and part of the middle cerebral artery region. Magnetic resonance angiography (MRA) showed an occlusion of the left middle cerebral artery [
Figure 1:
(a) Magnetic resonance imaging diffusion weighted imaging at initial onset. Diffuse high-intensity signals are observed in the left corona radiata and middle cerebral artery. (b) Magnetic resonance angiography, left middle cerebral artery occlusion. (c and d) Digital subtraction angiography, left internal carotid angiography, (c) is the early phase, and (d) is the late phase. Occlusion of the left middle cerebral artery and collateral blood flow from the left anterior cerebral artery through the leptomeningeal anastomosis.
Clinical course
The patient was admitted to the hospital at 8:25 a.m. Based on the MRI findings, we determined that there was a mismatch between the perfusion area of the left middle cerebral artery and the high signal DWI lesion. An intravenous alteplase therapy was deemed appropriate without any cautionary or contraindicated items as per the guidelines. Alteplase was administered at 9:27 a.m. following the management of hypotension with nicardipine. The primary strategy was to pursue an endovascular recanalization.
Endovascular treatment
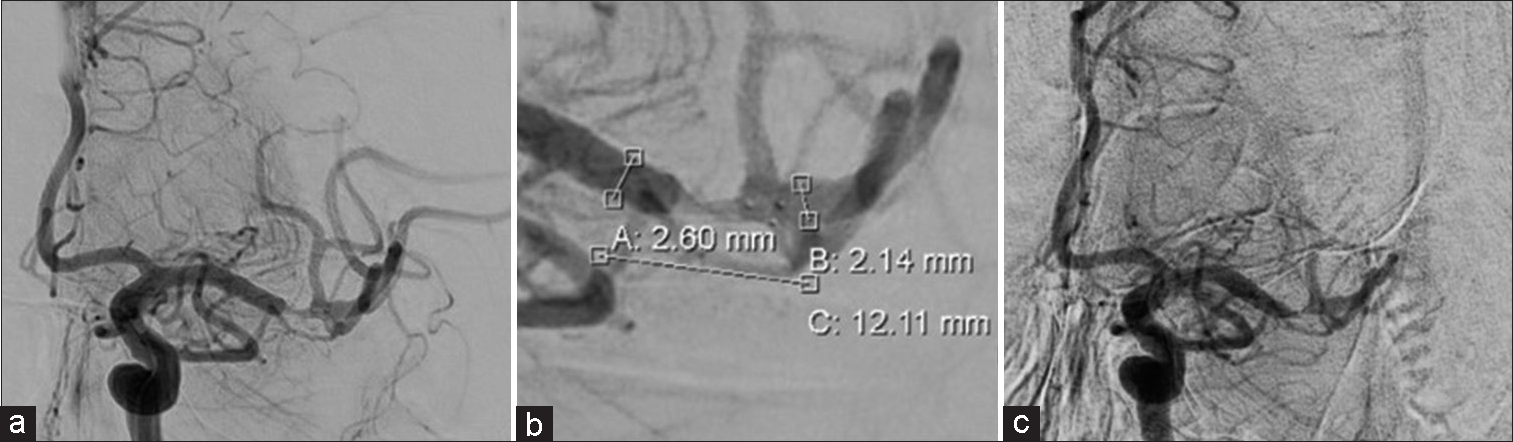
A 9 Fr sheath was placed in the right femoral artery, and a 9 Fr OPTIMO (Tokai Medical Products, Aichi, Japan) was guided into the left internal carotid artery. Internal carotid arteriography revealed that the left middle cerebral artery was distally occluded [
Figure 2:
(a and b) Left internal carotid angiography after Trevo deployment. Immediate flow restoration is confirmed. The lesion is at the distal middle cerebral artery. Dilation with Trevo is inadequate distal to the stenosis. (c) Left internal carotid angiography after Trevo retrieval. Recanalization has been achieved; however, severe stenosis remains, and peripheral perfusion is delayed.
The proximal and distal diameters of the stenosis were 2.6 mm and 2.1 mm, respectively [
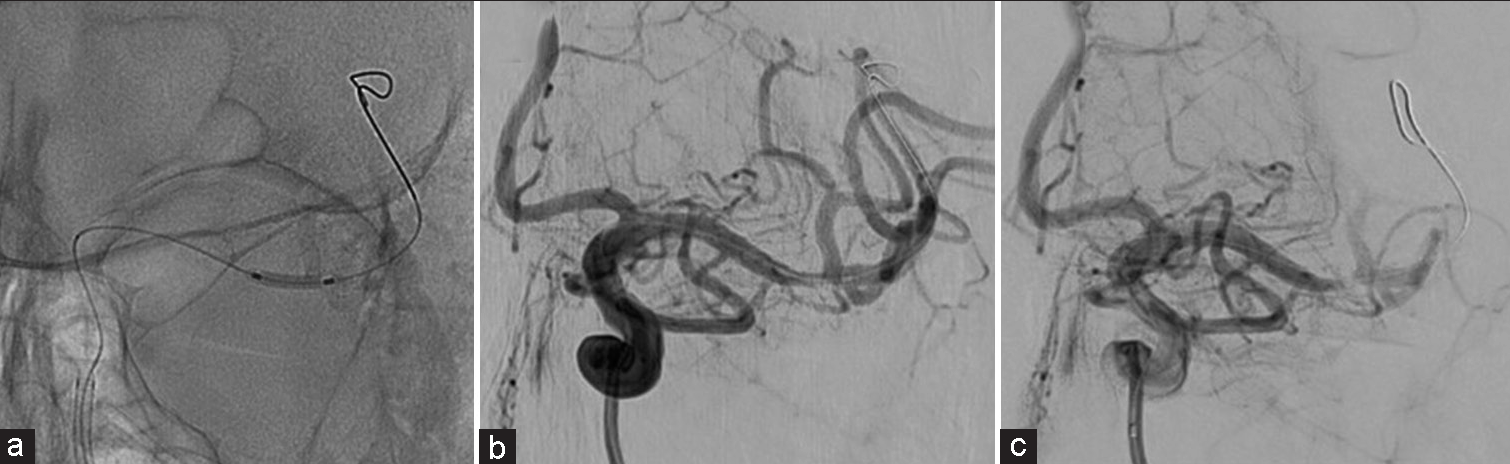
Crushed aspirin and clopidogrel (300 mg each) were administered orally. A 6 Fr Cerulean catheter DD6 113 cm (Medikit, Tokyo, Japan) was guided to the pyramidal segment of the internal carotid artery as a distal access catheter. Subsequently, a Gateway 2.0 × 12 mm monorail (Stryker, Kalamazoo, Michigan, USA) was placed at the stenosis region using a CHIKAI. A gradual dilatation and retraction were performed under nominal pressure [
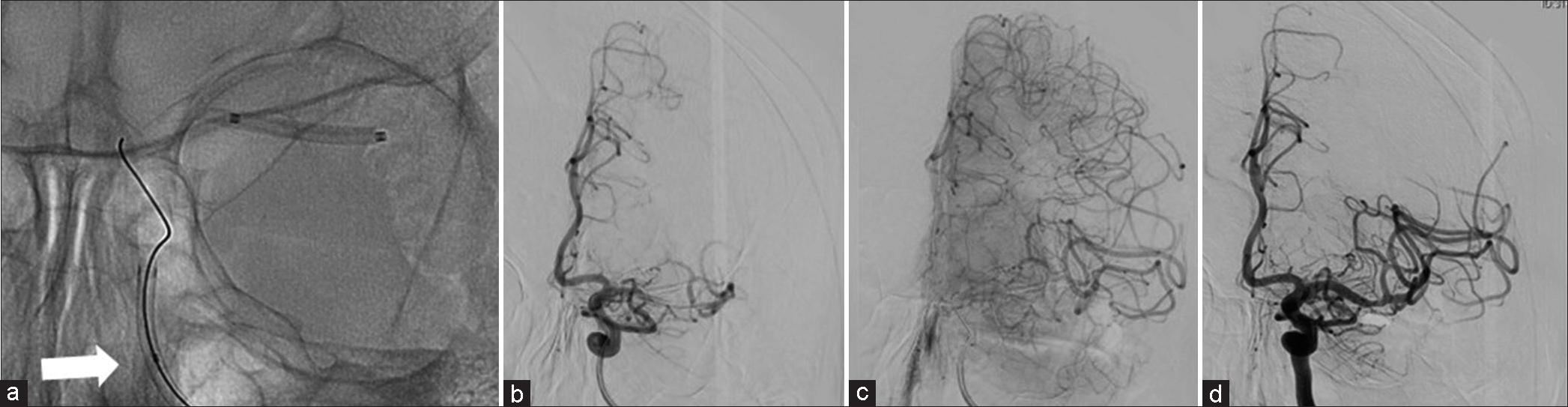
The strategy was to use a coronary perfusion balloon for prolonged angioplasty while maintaining the peripheral perfusion. Ryusei 2.5 × 20 mm monorail (Kaneka Medix, Osaka, Japan) was navigated toward the stenotic lesion. After dilating to 2 atm, the wire was drawn anterior to the proximal perfusion hole, and the perfusion lumen was subsequently unsealed [
Figure 4:
(a) Ryusei 2.5 × 20 mm monorail (2 atm) inflated at the center of the lesion. The white arrow indicates the perfusion marker. (b and c): Left internal carotid angiography during Ryusei inflation. (b) is the early phase, (d) is the late phase. Perfusion lumen enables the blood flow to traverse toward the distal of the balloon. (d) After angioplasty at Ryusei, the stenosis has improved.
The angiography performed in this state showed that peripheral perfusion was achieved using balloon dilation [
Heparin was administered while monitoring activated clotting time (ACT) during the procedure, with a minimum ACT value of 200. The total dose was 6,000 units.
Postoperative course
The patient’s symptoms resolved to an NIHSS score of 0 on the day after the procedure. Postoperative echocardiography and electrocardiography revealed no evidence of cardiogenic cerebral embolism. Based on the intraoperative findings, the patient was diagnosed with ICAD. Postoperatively, aspirin 100 mg and clopidogrel 75 mg prescriptions were continued, and cilostazol 200 mg and atorvastatin 10 mg were added. Postoperative MRI showed a clearly defined high signal DWI without significant enlargement of the infarcted area [
Figure 5:
(a) Magnetic resonance imaging diffusion weighted imaging on day 7. High-intensity signals are indicated in the left corona radiata. There is no enlargement compared to preoperative. (b) Magnetic resonance angiography on day 7. Mild stenosis is observed in the left middle cerebral artery; however, the recanalization findings are maintained. (c) Magnetic resonance angiography 3 months postoperatively. Improvement of the stenotic lesion is observed.
The patient was discharged without a neurological deficit on the 7th day.
Clopidogrel medication was discontinued at discharge. Three months later, the MRA indicated an improvement in the stenosis [
An ethical measure for off-label use
An application for an unapproved new medical device was submitted to the hospital’s committee for the evaluation of highly difficult new medical technology, and approval was obtained. Furthermore, during patient consultations regarding surgical procedures, we explicitly conveyed that we may employ nonconforming medical devices if adequate substitutions proved arduous and secured informed consent accordingly.
DISCUSSION
This case involved an intracranial LVO with a severe and sudden onset. The MRI DWI showed that the infarction was confined to a part of the perfusion area at the middle cerebral artery, suggesting a clinical diffusion mismatch or perfusion-diffusion mismatch, which required acute reperfusion therapy. There was no evidence of atrial fibrillation, other arrhythmias, or thrombosis on MRI, suggesting a cardiogenic cerebral embolism and the etiology of the occlusion was unknown at this point.
A preoperative diagnosis of ICAD was speculated based on the calcification on computed tomography and thrombotic findings on MRI magnetic susceptibility-enhanced images,[
The digital subtraction angiography findings, in this case, revealed the development of a collateral blood flow through the leptomeningeal anastomosis, indicating the potential presence of ICAD. Initially, the lesion was crossed with a microcatheter, and a stent retriever was deployed. Following the stent retriever deployment, angiography revealed an immediate flow restoration with no evidence of thrombus in the stent or poor stent dilation, thereby suggesting a diagnosis of ICAD.
No effective endovascular therapy has been reported for LVO caused by ICAD. Tsang et al. reviewed 11 articles and 1,967 cases of thrombectomy for intracranial LVO, regardless of the pathophysiology.[
Furthermore, most thrombectomy approaches in the evaluated studies involved the use of a stent retriever as the primary option. In the present case, as the etiology was difficult to predict, the deployment of Trevo with high strut visibility allowed visualization of the lesion and a temporary restoration of the blood flow. This facilitated the establishment of the etiology, and it was highly probable that an additional treatment would be required after thrombectomy, as previously reported.[
A minute volume of thrombus volume was retrieved using the stent retriever, and the subsequent angiography revealed a residual severe stenosis, as predicted. Therefore, the administration of antiplatelet agents and angioplasty were deemed necessary.
In Japan, the angioplasty devices indicated for acute intracranial lesions include Gateway and Unryu xp (Kaneka Medix, Osaka, Japan). These two devices possess slender and pliant tips and are attainable with balloon diameters as minute as 1.5 mm. In this case, we selected a 2 mm diameter Gateway, which was slightly slenderer than the diameter of the dilated site. However, this treatment was insufficient, and an elastic recoil manifested after the angioplasty procedure.
Two options were considered at this point. One was stenting, and the other was redilation using different balloons. The use of a Wingspan stent (Stryker, Kalamazoo, MI, USA), an aneurysmal stent, and a coronary stent was considered, although all stents would be used off-label. Perioperative complications in the stenting and aggressive medical management for preventing recurrent stroke (SAMMPRIS) trial[
In a study by Mori et al.[
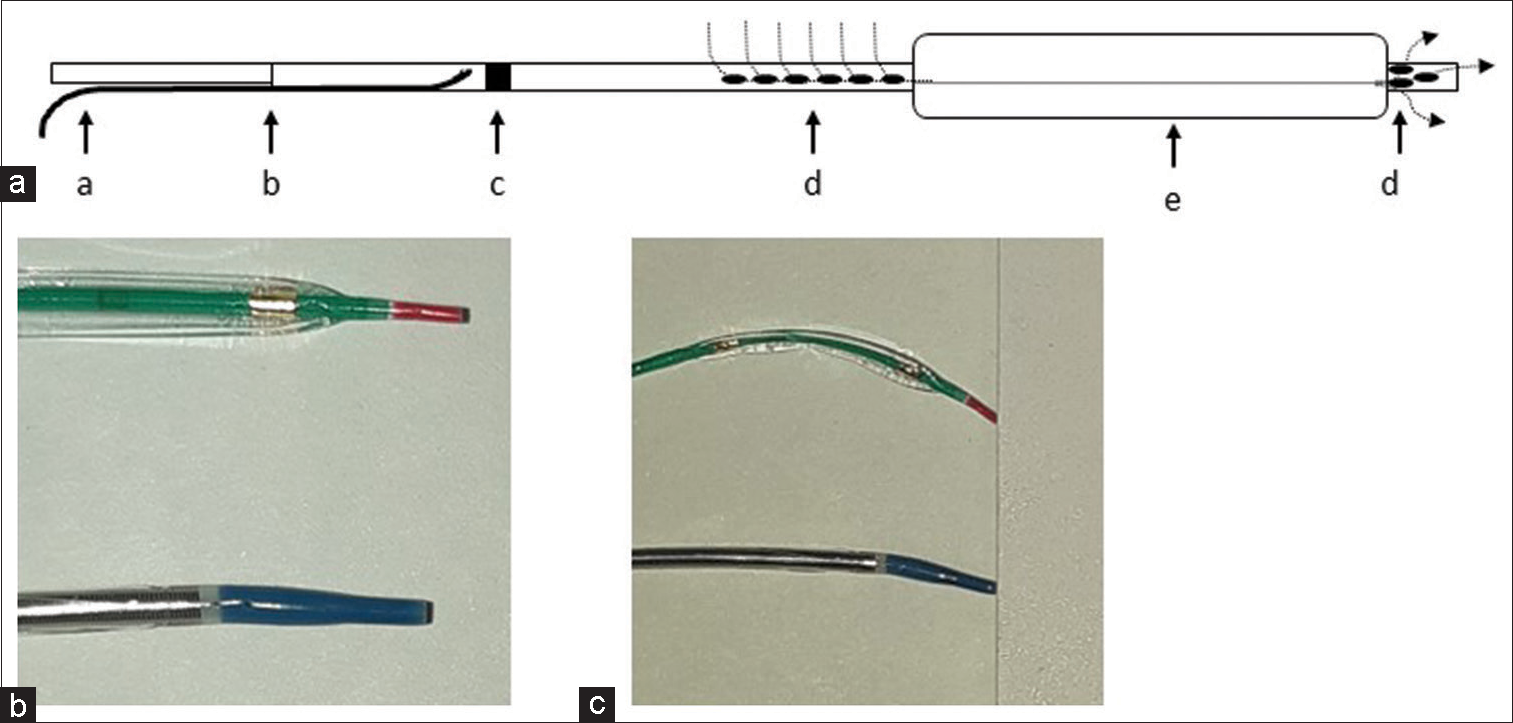
Based on these reports, we attempted a prolonged dilation procedure using different balloon catheters. Ryusei is a perfusion balloon catheter used for coronary angioplasty procedures; its structure is shown in [
Figure 6:
(a) Schematic of Ryusei balloon inflation. a: micro guidewire; b: guidewire entrance; c: perfusion marker; d: perfusion hole; e: balloon; dotted arrow: blood flow dynamics during balloon inflation. (b) Tip shape of both catheters: top, Gateway 2.0 × 12 mm; Bottom, Ryusei 2.5 × 20 mm. (c) Tip flexibility of both catheters.
As the minimum balloon diameter of this device was 2.5 mm, the dilatation conducted in this case was restricted to 2 atm, which resulted in an approximate dilation diameter of 2.35 mm. The subsequent imaging demonstrated satisfactory peripheral perfusion through the perfusion lumen [
We demonstrated a successful recanalization using a perfusion balloon. However, this device is intended for use in the coronary arteries, and its distal end exhibits greater thickness and reduced flexibility than cerebrovascular balloons [
It should be noted that the perfusion lumen is <1 mm in diameter, and there was a risk of thrombus formation or occlusion inside the lumen. Adequate antithrombotic therapy, confirmatory angiography, and heparin flushing every 5 min during balloon occlusion were used to prevent this.
In this case, the patient underwent a conventional mechanical thrombectomy for LVO. During the procedure, ICAD was identified, prompting treatment with antiplatelet agents and an angioplasty using a cerebrovascular balloon. However, this method is resistant to cerebrovascular balloon treatment. As such, the perfusion balloon was utilized with the utmost care, eventually resulting in favorable recanalization and outcomes.
Although it is off-label and requires proficiency, a perfusion balloon can potentially be used as an effective treatment for LVO due to refractory ICAD.
CONCLUSION
At present, there is no established endovascular treatment for LVO secondary to ICAD. Treatment with antiplatelet agents and angioplasty with cerebrovascular balloons are frequently insufficient.
The coronary perfusion balloons may serve as a potential therapeutic option for refractory ICAD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011. 365: 993-1003
2. Connors JJ, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: Evolution of technique and short-term results. J Neurosurg. 1999. 91: 415-23
3. Enomoto Y, Taki K, editors. Blood pharmacology. Neuroendovascular therapeutics. Osaka: Medicus Shuppan; 2018. p. 240-3
4. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016. 387: 1723-31
5. Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke. 2016. 18: 96-101
6. Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998. 19: 1525-33
7. Ohman EM, Marquis JF, Ricci DR, Brown RI, Knudtson ML, Kereiakes DJ. A randomized comparison of the effects of gradual prolonged versus standard primary balloon inflation on early and late outcome. Results of a multicenter clinical trial. Perfusion Balloon Catheter Study Group. Circulation. 1994. 89: 1118-25
8. Suh HI, Hong JM, Lee KS, Han M, Choi JW, Kim JS. Imaging predictors for atherosclerosis-related intracranial large artery occlusions in acute anterior circulation stroke. J Stroke. 2016. 18: 352-4
9. Tsang AC, Lau KK, Tsang FC, Tse MM, Lee R, Lui WM. Severity of intracranial carotid artery calcification in intracranial atherosclerosis-related occlusion treated with endovascular thrombectomy. Clin Neurol Neurosurg. 2018. 174: 214-6
10. Tsang AC, Orru E, Klostranec JM, Yang IH, Lau KK, Tsang FC. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke. 2019. 50: 1460-6