- Department of Neurosurgery, University of California Davis, Sacramento, California, United States
- Department of Neurosurgery, Kaiser Permanente Medical Center, Sacramento, California, United States.
Correspondence Address:
Edwin Samuel Kulubya, Department of Neurosurgery, University of California Davis, Sacramento, California, United States.
DOI:10.25259/SNI_1099_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Edwin Samuel Kulubya1, Nina Yu1, Jose Antonio Castillo1, Huy Duong2. External carotid artery-radial artery-vertebral artery bypass for surgical treatment of radiculopathy caused by an extracranial vertebral artery aneurysm: A case report and review of the literature. 27-Jan-2023;14:29
How to cite this URL: Edwin Samuel Kulubya1, Nina Yu1, Jose Antonio Castillo1, Huy Duong2. External carotid artery-radial artery-vertebral artery bypass for surgical treatment of radiculopathy caused by an extracranial vertebral artery aneurysm: A case report and review of the literature. 27-Jan-2023;14:29. Available from: https://surgicalneurologyint.com/surgicalint-articles/12127/
Abstract
Background: Vertebral artery (VA) aneurysm is a rare etiology of cervical radiculopathy and there is a paucity of case reports described in the literature.
Case Description: We describe a case of a patient with no history of trauma presenting with a large right VA aneurysm at the C5–C6 level compressing the C6 nerve root and causing a painful radiculopathy. The patient underwent successful external carotid artery-radial artery-VA bypass followed by trapping of the aneurysm and decompression of the C6 nerve root.
Conclusion: VA bypass is an effective tool for treatment of symptomatic large extracranial VA aneurysms and a rare cause of radiculopathy.
Keywords: Cervical radiculopathy, Operative technique, Radial artery graft, Vertebral artery aneurysm
INTRODUCTION
Vertebral artery (VA) aneurysms are a rare cause of cervical radiculopathy and there are only a few cases described in the literature.[
CASE DESCRIPTION
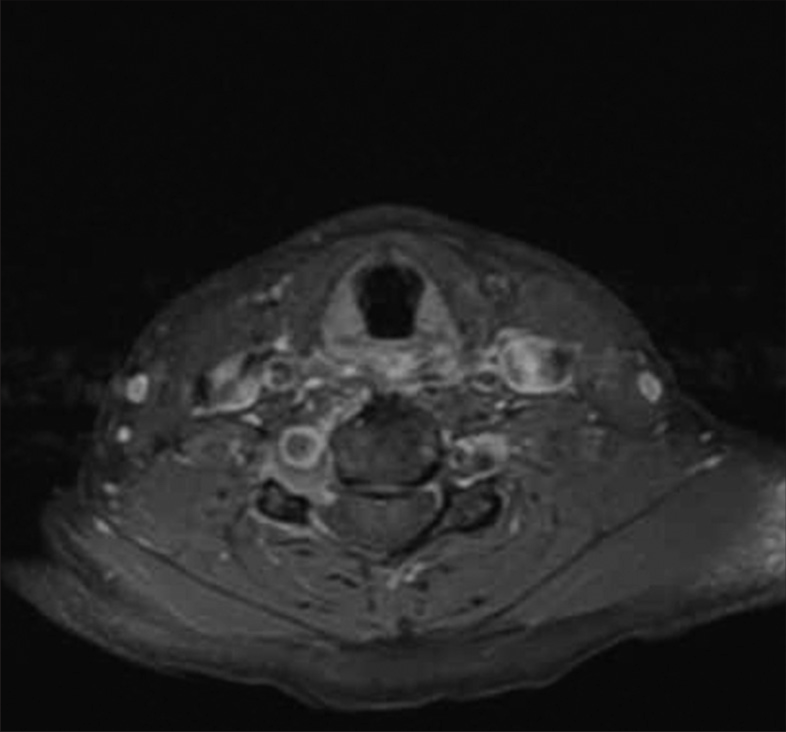
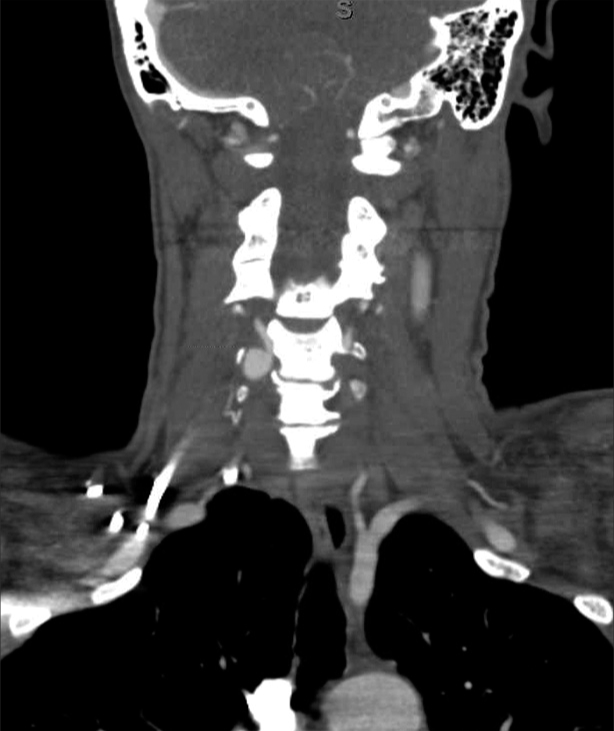
A 48-year-old right-handed female with no history of trauma or neck manipulation presented with debilitating pain in her right arm and hand for 2 months. Cervical spine MRI suggested foraminal stenosis of the C6 nerve but not secondary to a herniated disk or osteophyte. Bony remodeling was noticed through thinning of the right transverse process [
Our endovascular colleagues felt that a pipeline device may be limited due to the tortuosity and irregular appearance of the VA proximal to the aneurysm. After discussions with the patient, she expressed interest in preserving her VA and agreed to a surgical option which included a distal bypass and aneurysm trapping.
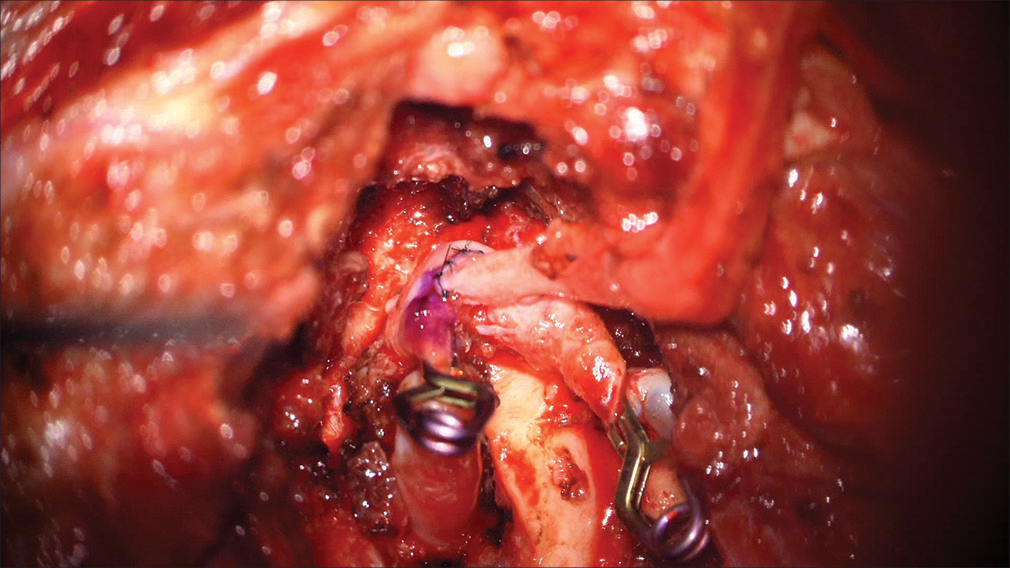
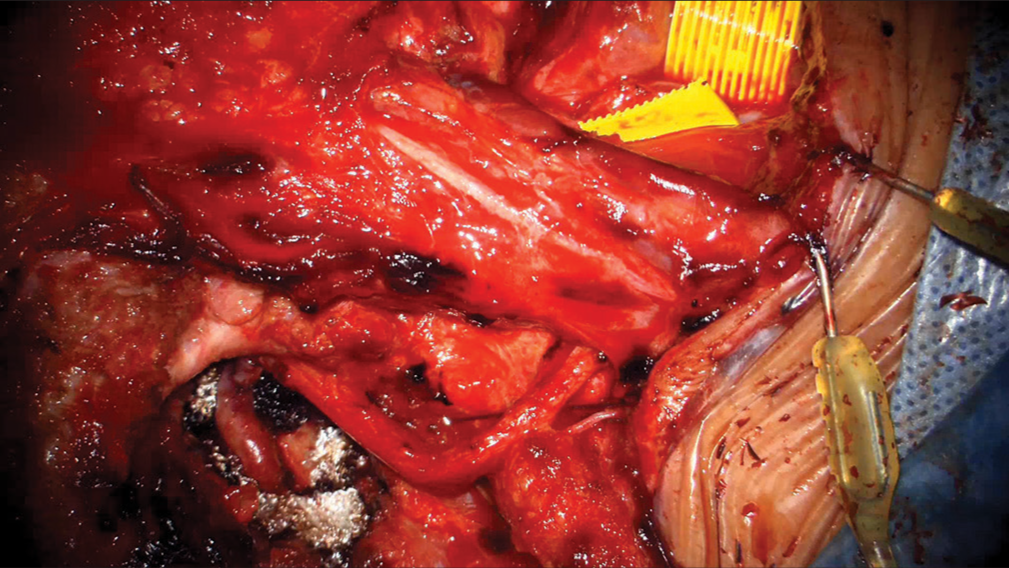
Under general anesthesia, the patient was positioned in a Mayfield head holder with pins and head turned to the left for a far lateral position. Somatosensory evoked potentials and motor evoked potentials were used throughout. A curved incision was made behind the ear extending into the anterior neck crease. The skin flap was, then, reflected anteriorly in the subplatysmal plane. The sternocleidomastoid muscle was identified, and anteriorly, the ECA was dissected out and a piece of background was placed. At the right suboccipital area the splenius capitis, the semispinalis capitis and the longissimus capitis were reflected inferiorly. The superior oblique and inferior oblique muscles were identified and dissected off their attachment to the transverse process of C1. The lateral mass and transverse process of C1 was exposed further and the V3 segment of the VA was identified superior to the C1 arch. The C1 right VA canal was opened using a drill and a Kerrison rongeur. The VA was, further, exposed and circumferentially dissected with all muscular branches ligated off and hemostasis obtained.
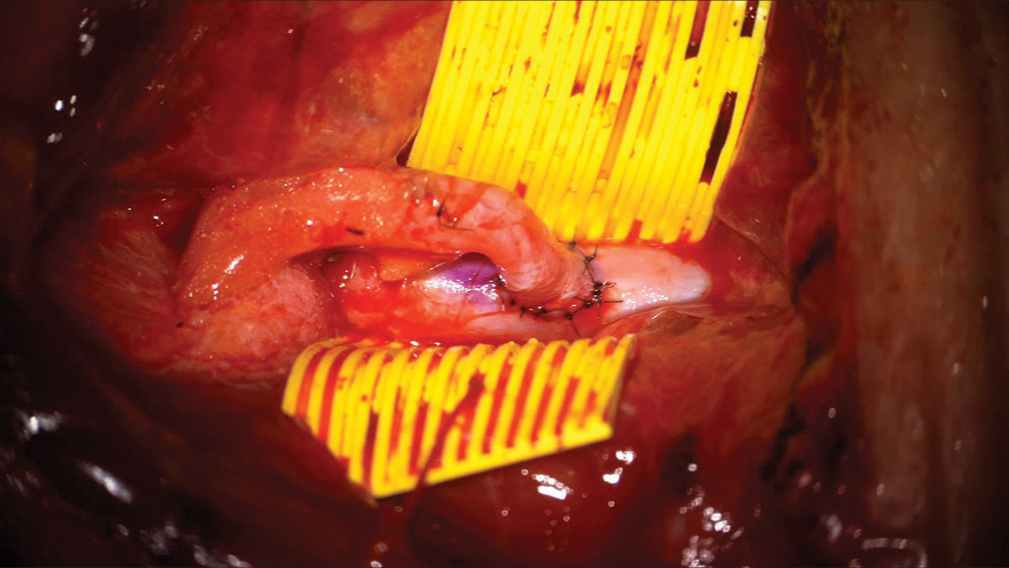
A harvested 12 cm radial artery (RA) graft was anastomosed to the V3 segment in a side-to-end fashion using 8-0 prolene with single interrupted sutures [
The patient was, then, positioned prone for the posterior cervical fusion. After exposure, the left C5, C6, and C7 lateral mass screws were placed using anatomical landmarks. On the right, the C5–6 and C6–7 facet joints were removed and the C6 transverse foramen was opened exposing the C6 nerve root and underlying aneurysm. The proximal end of the aneurysm was visualized and then clipped. Doppler confirmed no flow within the aneurysm. C5 and C7 lateral mass screws were, then, placed followed by bilateral rods. After irrigation, decortication, and placement of the bone graft, the wound was closed in multiple layers in a standard fashion. There were no operative complications.
At 12 months follow-up, the patient was doing well with no residual arm pain and resumed normal physical activity.
DISCUSSION
Cervical radiculopathy is commonly due to localized trauma and herniation of an intervertebral disc or degenerative changes which can lead to inflammation and compress a nerve root.[
The causes of VA aneurysms tend to be secondary to blunt or penetrating trauma, dissection with formation of a pseudoaneurysm,[
Across the literature, clinical presentation of EVA can vary from asymptomatic to symptomatic and depend on the location of the lesion.[
Once a vascular lesion is suspected, diagnostic modalities that provide visualization of vasculature should be adequately utilized as MRI findings have been mistaken for a neurofibroma, hemorrhage, or other mass lesion.[
Treatment options for EVA aneurysms are not standardized. Conservative modalities for treating VA lesions include observation and anticoagulation.[
Other types of bypass procedures have been noted in the literature including distal-VA-to-ICA, carotid artery-toVA bypass with a venous conduit, and autologous ECA-toVA bypass with grafts designed from the saphenous vein or RA.[
CONCLUSION
We describe a case report demonstrating a surgical technique new to the literature that successfully treated a severe right C6 radiculopathy secondary to a VA aneurysm. To the best of our knowledge, this is the first open ECA-VA bypass using the RA as a graft, followed by an aneurysm trapping for this indication. One-year post surgery, the patient demonstrated complete resolution of all initial symptoms. This case demonstrates that VA bypass is an effective tool for treatment of symptomatic large EVA aneurysms without sacrifice.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Arnold M, Bousser MG, Fahrni G, Fischer U, Georgiadis D, Gandjour J. Vertebral artery dissection: Presenting findings and predictors of outcome. Stroke. 2006. 37: 2499-503
2. Benny BV, Nagpal AS, Singh P, Smuck M. Vascular causes of radiculopathy: A literature review. Spine J. 2011. 11: 73-85
3. Börm W, Antoniadis G, Müller A, Richter HP. An Aneurysm of the cervicalvertebral artery causing radiculopathy-an unusual case. Klin Neuroradiol. 2003. 13: 117-21
4. Crum B, Mokri B, Fulgham J. Spinal manifestations of vertebral artery dissection. Neurology. 2000. 55: 304-6
5. Duan H, Mo D, Zhang Y, Zhang J, Li L. Carotid-vertebral artery bypass with saphenous vein graft for symptomatic vertebrobasilar insufficiency. Neurosurg Focus. 2019. 46: E8
6. Hadley MN, Spetzler RF, Masferrer R, Martin NA, Carter LP. Occipital artery to extradural vertebral artery bypass procedure. Case report. J Neurosurg. 1985. 63: 622-5
7. Hardmeier M, Haller S, Steck A, Lyrer P, Engelter S, Renaud S. Vertebral artery dissection presenting with fifth cervical root (C5) radiculopathy. J Neurol. 2007. 254: 672-3
8. Johnson-Mann C, Schonholz C, Vandergrift W, Elliott BM, Brothers T. Bypass and embolization for a vertebral artery aneurysm in a patient with Marfan syndrome. J Vasc Surg Cases. 2015. 1: 77-80
9. Kaplan SS, Ogilvy CS, Gonzalez R, Gress D, Pile-Spellman J. Extracranial vertebral artery pseudoaneurysm presenting as subarachnoid hemorrhage. Stroke. 1993. 24: 1397-9
10. Kiyohira M, Ishihara H, Oku T, Kawano A, Oka F, Suzuki M. Successful endovascular treatment for thrombosed giant aneurysm of the V1 segment of the vertebral artery: A case report. Interv Neuroradiol. 2017. 23: 628-31
11. Kocaeli H, Andaluz N, Choutka O, Zuccarello M. Use of radial artery grafts in extracranial-intracranial revascularization procedures. Neurosurg Focus. 2008. 24: E5
12. Kollmann D, Kinstner C, Teleky K, Wressnegger A, Koppensteiner R, Huk I. Successful treatment of a ruptured extracranial vertebral artery aneurysm with onyx instillation. Ann Vasc Surg. 2016. 36: 290.e297-10
13. Kubota H, Tanikawa R, Katsuno M, Izumi N, Noda K, Ota N. Vertebral artery-to-vertebral artery bypass with interposed radial artery or occipital artery grafts: Surgical technique and report of three cases. World Neurosurg. 2014. 81: 202.e1-8
14. Magnus W, Viswanath O, Viswanathan VK, Mesfin FB, editors. Cervical radiculopathy. StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. p.
15. Morasch MD, Phade SV, Naughton P, Garcia-Toca M, Escobar G, Berguer R. Primary extracranial vertebral artery aneurysms. Ann Vasc Surg. 2013. 27: 418-23
16. Negoro M, Nakaya T, Terashima K, Sugita K. Extracranial vertebral artery aneurysm with neurofibromatosis. Endovascular treatment by detachable balloon. Neuroradiology. 1990. 31: 533-6
17. Pataki Á, Nguyen DT, Nagy Z, Nardai S, Nemes B. Stent-graft treatment of a giant asymptomatic extracranial vertebral artery aneurysm: Case report and literature review. Ann Vasc Surg. 2022. 79: 442.e441-6
18. Peyre M, Ozanne A, Bhangoo R, Pereira V, Tadié M, Lasjaunias P. Pseudotumoral presentation of a cervical extracranial vertebral artery aneurysm in neurofibromatosis Type 1: Case report. Neurosurgery. 2007. 61: E658 discussion E658
19. Rifkinson-Mann S, Laub J, Haimov M. Atraumatic extracranial vertebral artery aneurysm: Case report and review of the literature. J Vasc Surg. 1986. 4: 288-93
20. Schievink WI, Piepgras DG. Cervical vertebral artery aneurysms and arteriovenous fistulae in neurofibromatosis Type 1: Case reports. Neurosurgery. 1991. 29: 760-5
21. Schittek A. Pseudoaneurysm of the vertebral artery. Tex Heart Inst J. 1999. 26: 9095
22. Shang EK, Fairman RM, Foley PJ, Jackson BM. Endovascular treatment of a symptomatic extracranial vertebral artery aneurysm. J Vasc Surg. 2013. 58: 1391-3
23. Silbert BI, Khangure M, Silbert PL. Vertebral artery dissection as a cause of cervical radiculopathy. Asian Spine J. 2013. 7: 335-8
24. Stavrinou LC, Stranjalis G, Stavrinou PC, Bontozoglou N, Sakas DE. Extracranial vertebral artery aneurysm presenting as a chronic cervical mass lesion. Case Rep Med. 2010. 2010: 938219
25. Sultan S, Morasch M, Colgan MP, Madhavan P, Moore D, Shanik G. Operative and endovascular management of extracranial vertebral artery aneurysm in Ehlers-Danlos syndrome: A clinical dilemma--case report and literature review. Vasc Endovascular Surg. 2002. 36: 389-92
26. Tabatabai G, Schöber W, Ernemann U, Weller M, Krüger R. Vertebral artery dissection presenting with ispilateral acute C5 and C6 sensorimotor radiculopathy: A case report. Cases J. 2008. 1: 139
27. Wu H, Wang M, Li K, Wang F. Treatment of extracranial vertebral artery aneurysm with flow diversion. World Neurosurg. 2020. 138: 328-31