- Department of Neurosurgery, Sapienza University of Rome, Rome, Italy.
Correspondence Address:
Anthony Kevin Scafa, Department of Neurosurgery, Sapienza University of Rome, Rome, Italy.
DOI:10.25259/SNI_997_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anthony Kevin Scafa, Marco Giugliano, Marco Gallo, Manolo Piccirilli. Extradural hemorrhagic spinal cavernous angioma in a paucisymptomatic child: A rare case with review of the current literature. 31-Mar-2022;13:123
How to cite this URL: Anthony Kevin Scafa, Marco Giugliano, Marco Gallo, Manolo Piccirilli. Extradural hemorrhagic spinal cavernous angioma in a paucisymptomatic child: A rare case with review of the current literature. 31-Mar-2022;13:123. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11496
Abstract
Background: Cavernous angiomas, also referred to as cavernous hemangiomas or cavernomas (CMs), are vascular malformative benign neoplasms that may develop in any part of the central nervous system. Spinal CMs are uncommon (overall incidence rate of 0.04–0.05%). Pure epidural CMs account for 1–2% of all spinal CMs and 4% of all spinal epidural tumors. Diagnosis is extremely rare in the pediatric age. To the best of our knowledge, only 10 cases have been described so far. The treatment of choice is microsurgical resection.
Case Description: We describe here the rare case of a cervicothoracic hemorrhagic spinal epidural cavernoma in a paucisymptomatic, 8-year-old female Bangladeshi child. C7–T2 laminectomy with excision of a scarcely defined, capsulated dark red lesion was performed with good recovery.
Conclusion: Spinal epidural cavernomas are rare. Childhood presentation is even rarer. The reason could be found in a greater “compliance” and to a rarer occurrence of acute bleeding in children, thus resulting in a delayed diagnosis. Surgical excision is the gold standard of treatment.
Keywords: Pediatric spinal cavernous angioma, Pediatric spinal cavernous hemangioma, Pediatric spinal epidural cavernoma
INTRODUCTION
Cavernous angiomas, also known as cavernous hemangiomas or cavernomas (CMs), are vascular malformative lesions consisting of a dense bundle of dilated capillary-like channels lacking intervening neural parenchyma. They may be found anywhere in the central nervous system (CNS), mostly in the brain where they are associated with an increased risk in symptomatic intracerebral hemorrhage, seizures, and focal neurological deficits. Spinal CMs are rare, particularly pure epidural ones. These are also extremely rare in the pediatric population, with only few cases described in the literature.
We report here the case of a child affected by a cervicothoracic hemorrhagic spinal epidural cavernoma in whom emergency surgery at diagnosis was performed. We also reviewed the available literature (using PubMed, Scopus, and Cochrane Library databases) to delineate the clinicoradiological features of the disease and the most frequently applied treatment options.
METHODS
Study design
The present paper consists of a case report and a systematic review of the literature conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Eligibility criteria
All written papers about pediatric extradural spinal CMs reporting demographical and clinical data, diagnostic workflow, treatment protocol, histological findings, potential postoperative complications, and prognosis were considered for eligibility. Articles lacking single-patient information were excluded together with surgical and radiological technical notes, abstracts from scientific meetings, and unpublished reports. Articles discussing anything other than spinal epidural CMs were considered “not eligible,” along with those in which the disease was reported in an adult (>18 years).
Papers in languages other than English were considered for eligibility through the analysis of the abstract (only in cases in which it was available and English written).
Information sources and search strategy
The systematic review of the literature was conducted on three different online medical databases (PubMed, Scopus, and Cochrane Library), using as search terms “child,” “childhood,” “children,” “spine,” “spinal,” “extradural,” “epidural,” “cavernous malformation,” “cavernous angioma,” “cavernous hemangioma,” “cavernoma,” and combined with the Boolean operators “OR” and “AND” ([Title/Abstract]). The last search was conducted on December 31, 2021, and went back as far as data were available. The search strategy is summarized in [
Data collection process
Abstracts and full texts were independently screened by two authors (A.K.S. and M.G.), and any discordance was solved by consensus with a third senior author (M.P.).
CASE REPORT
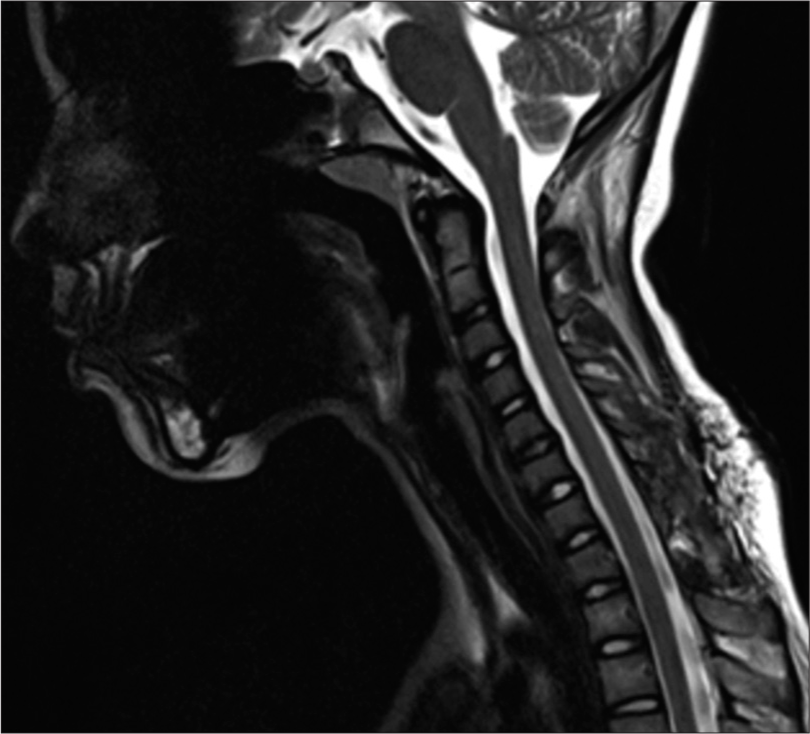
An 8-year-old female Bangladeshi child was referred to the pediatric emergency department of our institution with a 7-day history of thoracic pain with progressive nontraumatic cervicodorsal discomfort and constipation in the past 48 h. A medical history was unremarkable except for a precocious puberty under evaluation. Physical examination on admission showed a marked pain-induced limitation in neck movements with tenderness on palpation of cervicodorsal muscles. Abdominal distension was also detected. Neurological examination was otherwise negative. The child was admitted to the pediatric unit. Despite oral analgesia, the pain gradually increased. After 3 days of essentially normal investigations, a magnetic resonance imaging (MRI) of the cervicodorsal spine [
Figure 2:
Preoperative MRI. (a) Sagittal section, C+ T1WI showing a nonenhanced iso-hyperintense epidural mass compressing the thecal sac from behind. Note the edema of the paravertebral soft tissues and the straightening of the cervical spine from antalgic contracture. (b) Sagittal section, T2WI showing hypointense signal of the lesion. (c) Axial section, T2WI showing T1–T2 foraminal extension of the lesion.
RESULTS
After duplication removal, 148 papers were screened for this systematic review. Forty-six papers were excluded for the following reasons according to our exclusion criteria: 38 since “not pertinent,” while eight because “not retrieved;” 102 full-text papers were, therefore, evaluated. Among these, 93 papers were excluded according to our inclusion criteria (76 since discussing “adult presentation,” 17 due to scarcity of data), and three “foreign” papers were similarly excluded lacking a complete, English written abstract. Six papers (reporting eight cases) were finally included and analyzed. Two studies (each reporting one case) were found through further search (references/Google) and were similarly included.
Findings related to the reported cases are shown in [
DISCUSSION
Spinal epidural hematoma (SEH) is a relatively rare, but potentially disabling disease. First described by Jackson in 1869,[
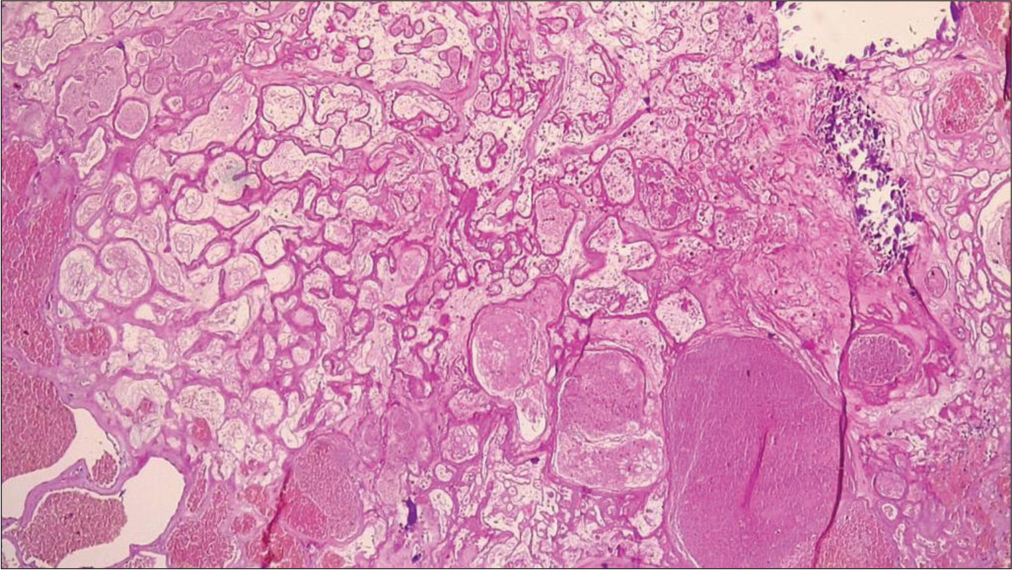
CMs are vascular malformative benign neoplasms that may affect any part of the CNS. They consist of closely packed endothelium-lined vascular channels within a collagenous stroma. Some of these vessels are partially or completely thrombosed and contain hemorrhage in different stages of evolution. Most of these lesions occurs in the brain, with 63–90% in the supratentorial compartment and 7.8–35.8% located infratentorially.[
Less than 100 cases have been reported.[
Spinal CMs are frequently diagnosed in women between the third and the sixth decades. Diagnosis is extremely rare in the pediatric age (<18 years), though they are fundamentally congenital. To the best of our knowledge, only 10 cases have been described hitherto [
Overall mean age was 8.9 years (range 1.7–14 years). Females were slightly more frequently affected than males (F/M ratio = 1.8:1). The lesions extended between 1 and 3 vertebrae in 8 cases (72.7%); the extension was greater in the remaining 3 cases (27.3%), with, in one case, 12 vertebrae involved. Cervicothoracic junction was the preferred site (n = 6, 54.5%). Pure thoracic location was found in 2 cases (18.2%), while thoracolumbar and sacral locations were “extraordinary.” This may be specific of the pediatric age since spinal CMs usually occur in the thoracic and lumbar spine in adulthood.[
Clinical status at the time of first observation and follow-up was assessed using a modified Neuro-Grade (NG) scale previously reported in other studies.[
Certain MRI features may be useful for the differential diagnosis. T1-weighted images usually show a homogeneous iso- or hypointense signal intensity (though hypersignal has also been described, like in cases 2, 3, 7, and in our case [
The treatment of choice for spinal CMs is microsurgical en bloc resection of the lesions.[
CONCLUSION
Spinal epidural cavernomas are uncommon lesions. Diagnosis is rarely made in childhood, and it is important to rule them out even in case of chronic or subacute onset of spinal pain. Surgical excision is the gold standard of treatment.
STATEMENTS
Statement of ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from the parents of the patient for PUBLICATION OF THIS CASE REPORT AND ANY ACCOMPANYING IMAGES. Ethical approval was not required for this study in accordance with national guidelines.
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Authors’ contributions
A.K.S. conceived and designed the analysis, collected the data, and wrote the paper. M.G.I and M.G.II collected the data with the first author. M.P. critically revised the work.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alvarez Sastre C, Martín-Gamero AP, Ortega FV. Paraplejia en lactante por hematoma extradural debido a sangrado de cavernoma espinal. Neurocirugía. 1999. 10: 367-71
2. Amhaz HH, Fox BD, Johnson KK, Whitehead WE, Curry DJ, Luerssen TG, Jea A. Postlaminoplasty kyphotic deformity in the thoracic spine: Case report and review of the literature. Pediatr Neurosurg. 2009. 45: 151-4
3. Aoyagi N, Kojima K, Kasai H. Review of spinal epidural cavernous hemangioma. Neurol Med Chir. 2003. 43: 471-6
4. Cabral AJ, Barros A, Aveiro C, Vasconcelos R. Spontaneous spinal epidural haematoma due to arteriovenous malformation in a child. BMJ Case Rep. 2011. 2011: bcr0220113875
5. Caruso R, Martines V, Marrocco L, Piccione E, Wierzbicki V, Lombardi M. Case report: An epidural cavernous hemangioma mimicking a dumbbell-shaped neuroma. Int J Surg Case Rep. 2021. 84: 106069
6. Cho JH, Chung YN, Wang KC, Cho BK, Cho RK. Spinal cavernous hemangioma causing sudden paraplegia in a 23-month-old kid. J Korean Neurosurg Soc. 2006. 40: 273-6
7. Domenicucci M, Marruzzo D, Pesce A, Raco A, Missori P. Acute spinal epidural hematoma after acupuncture: Personal case and literature review. World Neurosurg. 2017. 102: 695.e11-4
8. Domenicucci M, Ramieri A, Ciappetta P, Delfini R. Non-traumatic acute spinal subdural hematoma: Report of five cases and review of the literature. J Neurosurg. 1999. 91: 65-73
9. Domenicucci M, Ramieri A, Paolini S, Russo N, Occhiogrosso G, Di Biasi C. Spinal subarachnoid hematomas: Our experience and literature review. Acta Neurochir (Wien). 2005. 147: 741-50
10. Domenicucci M, Mancarella C, Santoro G, Dugoni DE, Ramieri A, Arezzo MF. Spinal epidural hematomas: Personal experience and literature review of more than 1000 cases. J Neurosurg Spine. 2017. 27: 198-208
11. Dugoni DE, Mancarella C, Landi A, Tarantino R, Ruggeri AG, Delfini R. Post laminoplasty cervical kyphosis-case report. Int J Surg Case Rep. 2014. 5: 853-7
12. Feng J, Xu YK, Li L, Yang RM, Ye XH, Zhang N. MRI diagnosis and preoperative evaluation for pure epidural cavernous hemangiomas. Neuroradiology. 2009. 51: 741-7
13. Giombini S, Morello G. Cavernous angiomas of the brain. Account of fourteen personal cases and review of the literature. Acta Neurochir. 1978. 40: 61-82
14. Goodarzi A, Clouse J, Capizzano T, Kim KD, Panchal R. The optimal surgical approach to intradural spinal tumors: Laminectomy or hemilaminectomy?. Cureus. 2020. 12: e7084
15. Jackson R. Case of spinal apoplexy. Lancet. 1869. 2: 5-6
16. Jonas AF. Spinal fractures, opinions based on observations of sixteen operations. JAMA. 1911. 9: 859-63
17. Joshi GK, Krishna KN, Krishna DG, Murthy GK, Herur A, Karnam SV. Dorsal spinal epidural cavernous angioma; A case report. Asian J Neurosurg. 2021. 16: 144-9
18. Khalatbari MR, Abbassioun K, Amirjmshidi A. Solitary spinal epidural cavernous angioma: Report of nine surgically treated cases and review of the literature. Eur Spine J. 2013. 22: 542-7
19. Lee JW, Cho EY, Hong SH, Chung HW, Kim JH, Chang KH. Spinal epidural hemangiomas: Various types of MR imaging features with histopathologic correlation. AJNR Am J Neuroradiol. 2007. 28: 1242-8
20. Mühmer M, Bostelmann R, Sarikaya-Seiwert S, Schneiderhan M, Steiger HJ, Cornelius JF. Clinical and radiological presentation of spinal epidural haemangiomas: Clinical series in a tertiary care centre during a 10-year period. Eur Spine J. 2014. 23: 404-10
21. Naganawa T, Miyamoto K, Hosoe H, Suzuki N, Shimizu K. Hemilaminectomy for removal of extramedullary or extradural spinal cord tumors: Medium to long-term clinical outcomes. Yonsei Med J. 2011. 52: 121-9
22. Porter RW, Detwiler PW, Spetzler RF, Lawton MT, Baskin JJ, Derksen PT. Cavernous malformations of the brainstem: Experience with 100 patients. J Neurosurg. 1999. 90: 50-8
23. Raab P, Juergen K, Gloger H, Soerensen N, Wild A. Spinal deformity after multilevel osteoplastic laminotomy. Int Orthop. 2008. 32: 355-9
24. Richardson RR, Cerullo LJ. Spinal epidural cavernous hemangioma. Surg Neurol. 1979. 12: 266-8
25. Roman A, Filho PM, Manzato LB, De Carli F, Schwingel D, Oliveira TA. Spinal cavernous hemangioma in a pediatric patient-a case report. J Spine. 2015. p.
26. Santoro A, Piccirilli M, Bristot R, di Norcia V, Salvati M, Delfini R. Extradural spinal cavernous angiomas: Report of seven cases. Neurosurg Rev. 2005. 28: 313-9
27. Sarikaya-Seiwert S, Gierga K, Wessalowski R, Steiger HJ, Hänggi D. Solitary spinal epidural cavernous angiomas in children presenting with acute neurological symptoms caused by hemorrhage. J Neurosurg Pediatr. 2010. 5: 89-93
28. Sun I, Pamir MN. Spinal cavernomas: Outcome of surgically treated 10 patients. Front Neurol. 2017. 8: 672
29. Zevgaridis D, Büttner A, Weis S, Hamburger C, Reulen HJ. Spinal epidural cavernous hemangiomas. Report of three cases and review of the literature. J Neurosurg. 1998. 88: 903-8