- Department of Neurosurgery Dr. Soeradji Tirtonegoro General Hospital, Klaten, Central Java, Indonesia.
- Department of Pathology Anatomy, Dr. Soeradji Tirtonegoro General Hospital, Klaten, Central Java, Indonesia.
Correspondence Address:
Ayu Yoniko Christi, Department of Neurosurgery, Dr. Soeradji Tirtonegoro General Hospital, Klaten, Central Java, Indonesia.
DOI:10.25259/SNI_1055_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ayu Yoniko Christi1, Wisnu Baskoro1, Bidari Kameswari2, Irfaanstio Akbar Hakim1, Vega Sola Gracia Pangaribuan1, Andrianto Purnawan1. Extradural malignant peripheral nerve sheath tumor of the thoracic spine: A rare case report. 16-Nov-2021;12:560
How to cite this URL: Ayu Yoniko Christi1, Wisnu Baskoro1, Bidari Kameswari2, Irfaanstio Akbar Hakim1, Vega Sola Gracia Pangaribuan1, Andrianto Purnawan1. Extradural malignant peripheral nerve sheath tumor of the thoracic spine: A rare case report. 16-Nov-2021;12:560. Available from: https://surgicalneurologyint.com/surgicalint-articles/11229/
Abstract
Background: Malignant peripheral nerve sheath tumors (MPNSTs) typically found in the trunk, limbs, head, and neck represent 3–10% of all soft-tissue sarcomas. Although they typically originating from peripheral nerve Schwann cells, 2–3% arise from the spinal nerves and may be found within the spinal canal. Here, we present a 43-year-old male with an extradural thoracic MPNST contributing to marked cord compression and a progressive paraparesis.
Case Description: A 43-year-old male presented with a progressive paraparesis of 16 months’ duration. The MRI showed a posterior T2-T4 extradural tumor in the thoracic spine resulting in significant cord compression. Following a T2-T4 laminectomy and gross total excision of the epidural mass, the patient regained modest neurological function. Immunohistochemistry staining supported the diagnosis of thoracic spinal MPNST.
Conclusion: Rarely, spinal MPNST can be considered amongst the differential diagnoses of an extradural spinal tumor. In this case, gross total excision of a posterior T2-T4 epidural MPNST resulted in improvement in the patient’s original paraparesis. Notably, immunohistochemistry staining helped confirm the diagnosis of a MPNST.
Keywords: Epidural mass, Extradural tumor, Histopathology, Malignant peripheral nerve sheath tumors, Spinal tumor
INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNSTs) are rare (i.e. 3–10%; incidence 0.001% in general population) soft-tissue sarcomas that typically arise from major or minor peripheral nerve branches or sheaths of peripheral nerve fibers (i.e., derived from Schwann cells or pluripotent cells of neural crest origin).[
CASE REPORT
A 43-year-old male presented with 16 months of a progressive paraparesis to final paraplegia, hypoesthesia below the T10 sensory level, and loss of sphincter function. Interestingly, there was no past medical history or family history of neurofibromatosis. All lab studies were normal.
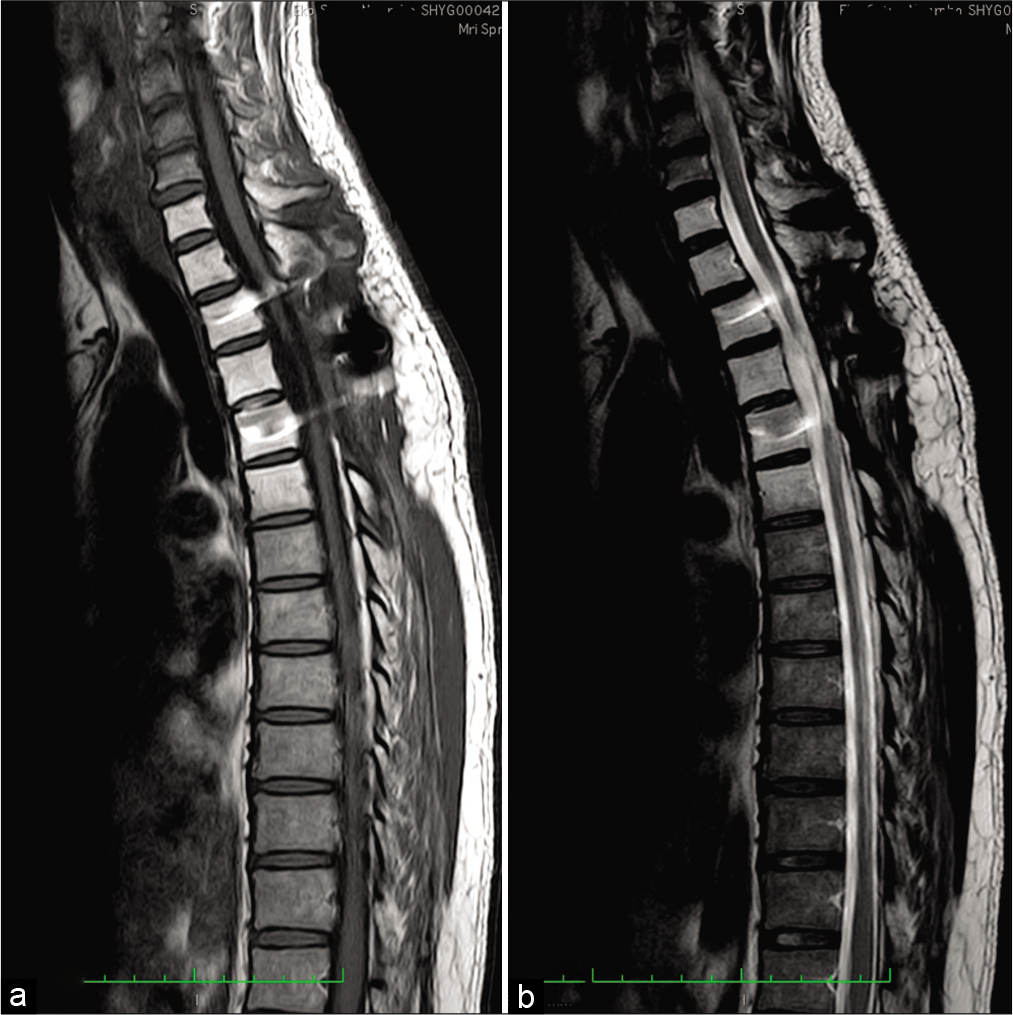
MRI study results
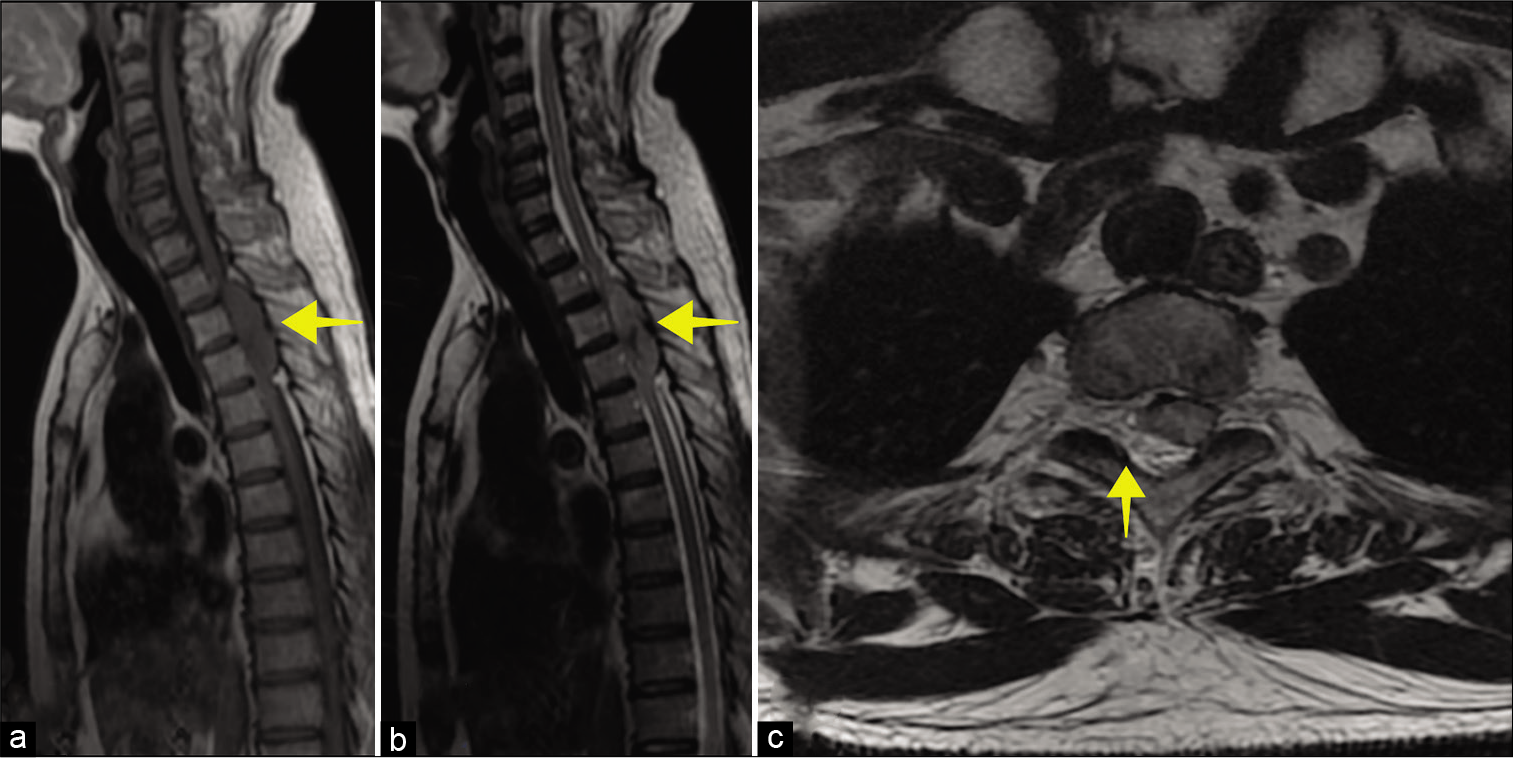
A whole spine MRI revealed a posterior isointense soft-tissue mass, measuring 69.5 × 16 mm at the T2-T4 levels that markedly compressed the cord [
Figure 1:
MRI of the thoracic spine. (a) Sagittal T1 and (b) sagittal T2-weighted image shows isointense extradural mass (yellow arrow) in thoracic spinal canal lying from T2 until T4 levels. (c) Axial T2-weighted imaging shows severe thoracic canal narrowing due to compression by the mass (yellow arrow).
Surgery
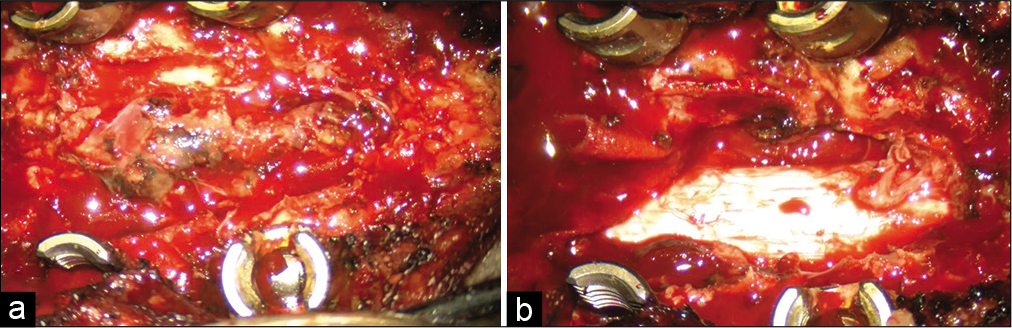
The patient underwent a T2-T4 laminectomy for posterior extradural tumor resection followed by pedicle screw stabilization. The lesion had a well-defined border and was removed in a piecemeal fashion; the dura remained intact [
Histopathological evaluation
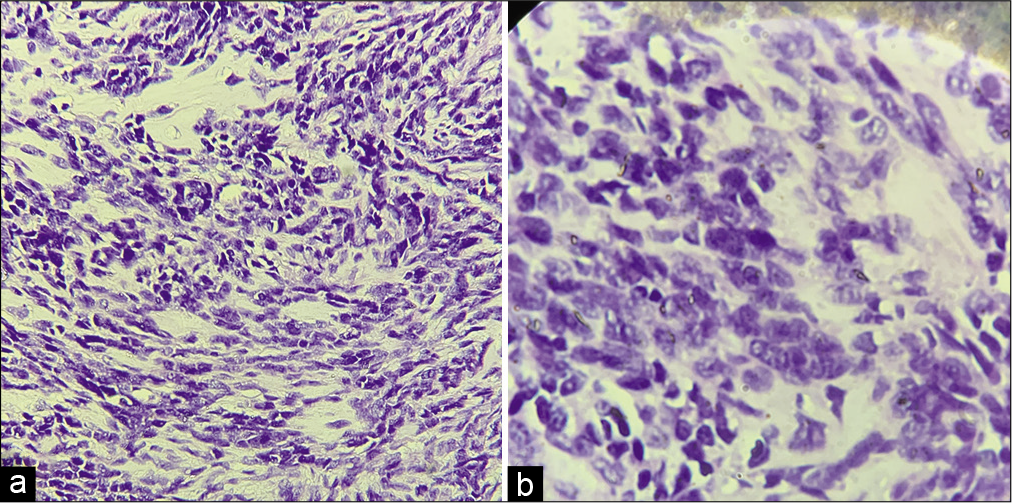
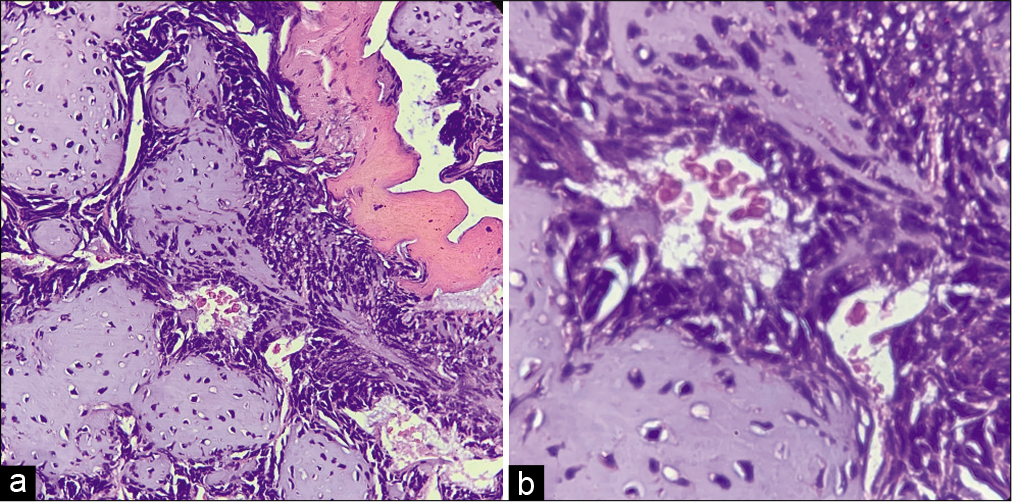
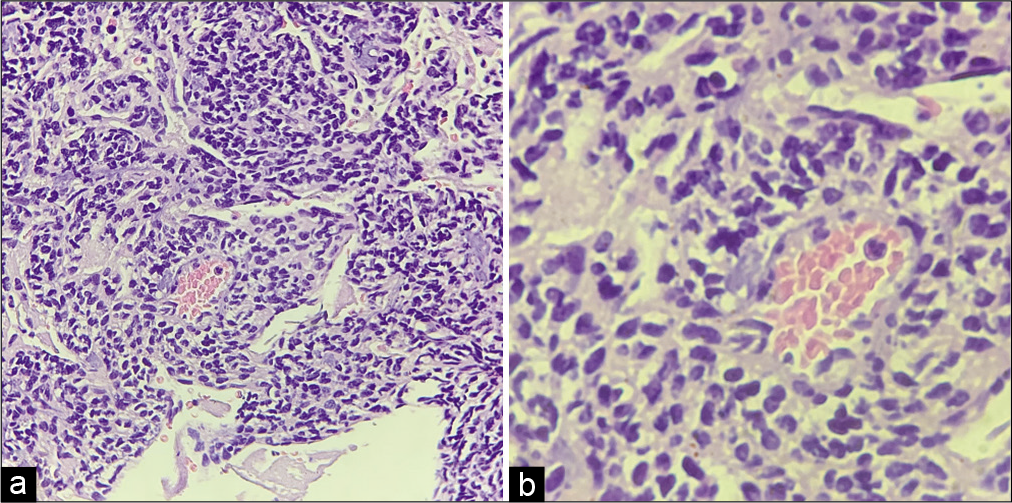
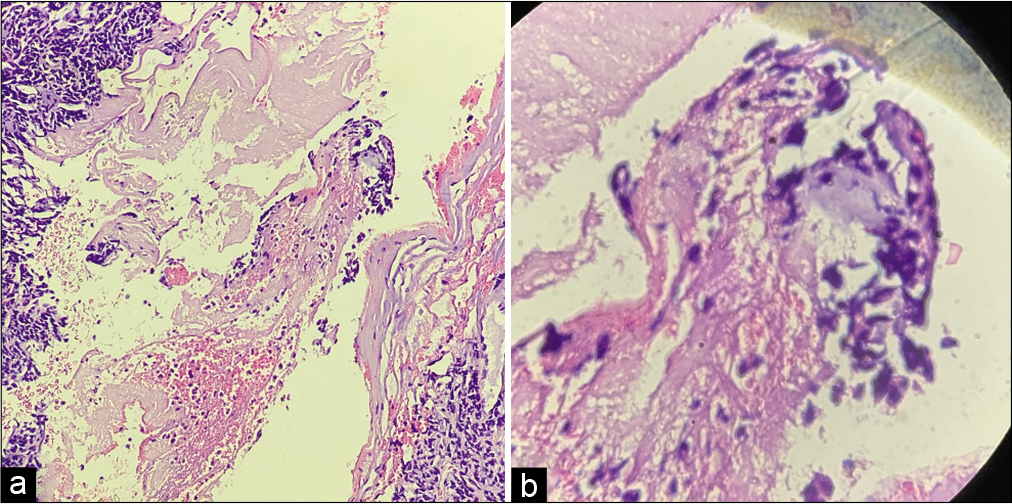
On gross analysis, the specimen consisted of an aggregate of brownish-white soft-tissue fragments with a spongy consistency (i.e., weight 15 g; largest measurements were 1.5 × 2 × 0.6 cm). Histopathology revealed brisk mitotic activity with the cellular area consisting of spindle nuclei arranged in a palisading pattern [
Postoperative course and radiation treatment
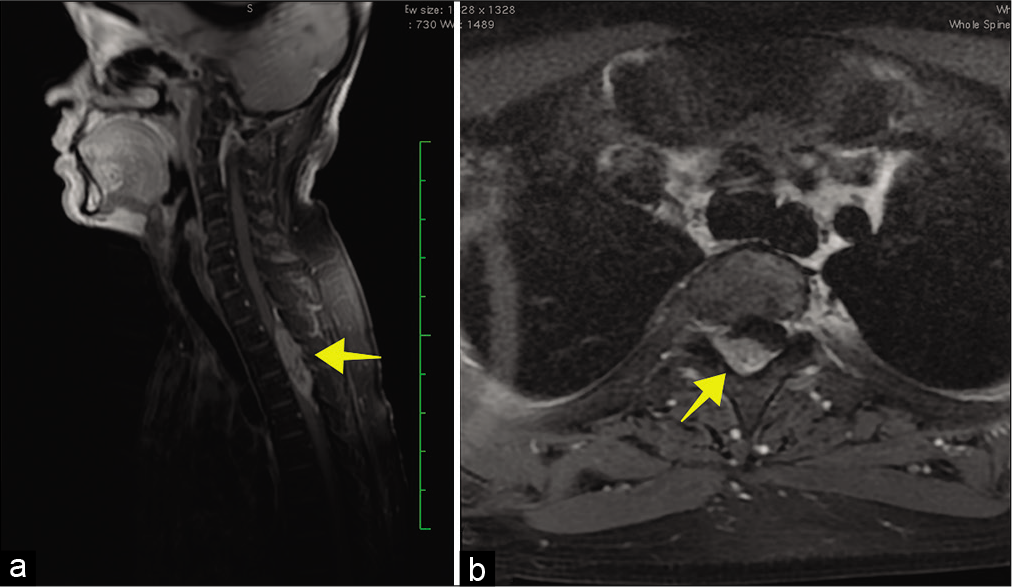
Postoperatively, the numbness in the lower extremities largely resolved but the motoric deficit improved only marginally. The patient was referred to an oncologist and received adjuvant radiotherapy with total dose of 45 Gray divided into 22 fractions. Six months later, after completion of radiotherapy, the thoracic spine MRI without contrast demonstrated cord swelling at the T2-T3-T4 levels [
DISCUSSION
MPNST is a rare form of sarcoma, accounting for only 10% of all soft tissue sarcomas.[
Despite gross total resection of these lesions, there is still a local recurrence rate of 32–65% after median intervals of 5–32.2 months.[
Status of postoperative radiation therapy
At present, postoperative radiotherapy is recommended by the oncology consensus group for MPNST. Adjuvant radiotherapy provides local control, and may delay the onset of recurrence, but has little effect on long-term survival.[
Overall prognosis of MPNST
Patients with MPNST have a poor prognosis. The 5-year survival rates range from 20 to 50%, and a < 20% for spinal MPNST.[
CONCLUSION
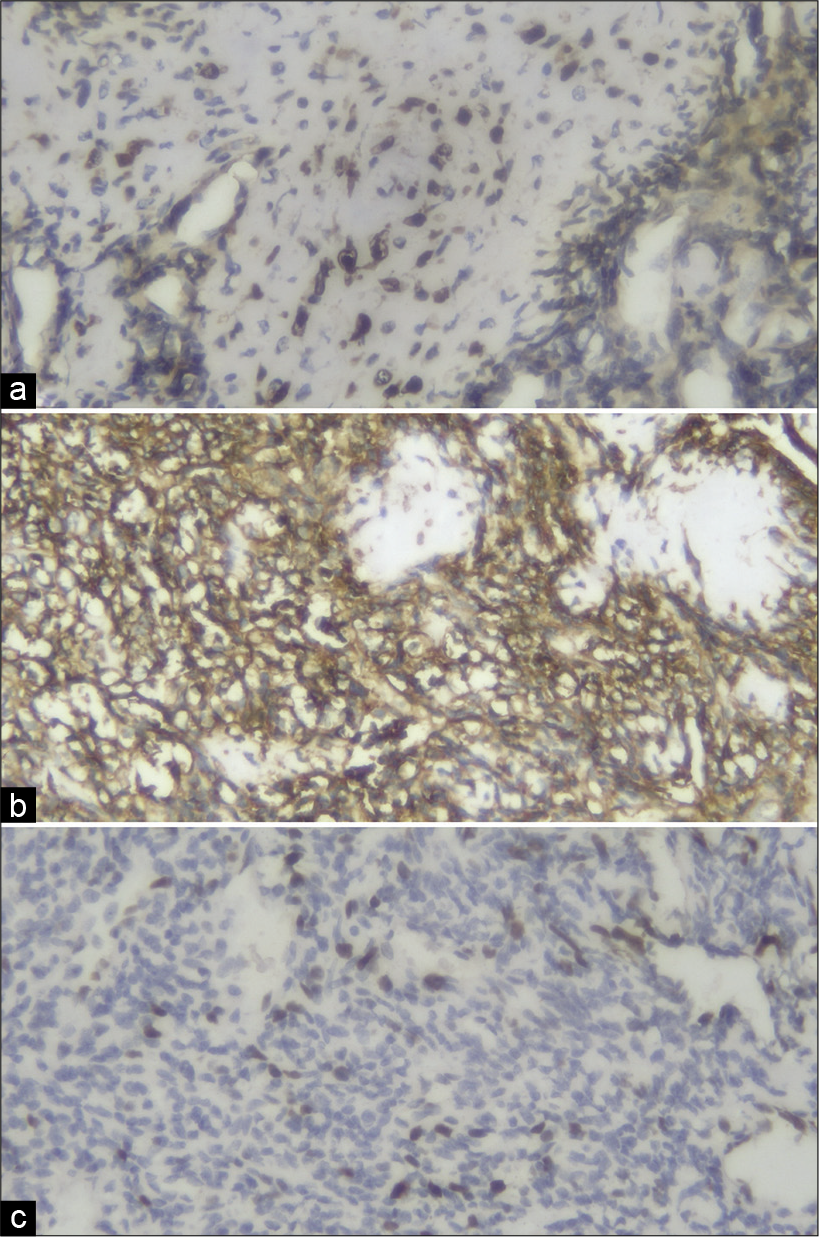
Spinal MPNST, although rare, should be considered among the differential diagnoses for extradural spinal tumors. Here, immunohistochemistry staining of S100, CD99, and Ki67 confirmed the diagnosis of a MPNST. Despite gross total tumor excision and adjuvant radiotherapy, these lesions have a high recurrence rate with poor long-term survivals.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledged Nadia Christina, M.D for planning and conducting the radiotherapy management for the patient.
References
1. Amirian ES, Goodman JC, New P, Scheurer ME. Pediatric and adult malignant peripheral nerve sheath tumors: An analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol. 2014. p. 609-16
2. Chou D, Bilsky MH, Luzzati A, Fisher CG, Gokaslan ZL, Rhines LD. Malignant peripheral nerve sheath tumors of the spine: Results of surgical management from a multicenter study. J Neurosurg. 2017. 26: 291-8
3. Gupta G, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Focus. 2007. 22: 1-8
4. Peterson MS, Waddell JK, Ebbert TL, Perry A, Berg LC. Malignant peripheral nerve sheath tumor within the spinal canal with apparent drop metastases. Hum Pathol Case Rep. 2018. 14: 88-91
5. Widemann BC. Current status of sporadic and neurofibromatosis Type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep. 2009. 11: 322-7
6. Winn HR.editors. Youmans and Winn Neurological Surgery. Philadelphia, PA: Elsevier; 2017. p.
7. Zhu B, Liu X, Liu Z. Malignant peripheral nerve sheath tumours of the spine: Clinical manifestations, classification, treatment, and prognostic factors. Eur Spine J. 2012. p. 897-904