- School of Medicine, Loma Linda University, Loma Linda, United States
- Department of Neurosurgery, Riverside University Health System, Moreno Valley, United States
- Department of Neurosurgery, Loma Linda University Children’s Hospital, Loma Linda, United States.
Correspondence Address:
Jessica Sawaya, Loma Linda University School of Medicine, Loma Linda, United States.
DOI:10.25259/SNI_27_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jessica Sawaya1, Paras Savla2, Tanya Minasian3. Extradural spinal cyst in a pediatric patient: A case report. 05-Apr-2024;15:123
How to cite this URL: Jessica Sawaya1, Paras Savla2, Tanya Minasian3. Extradural spinal cyst in a pediatric patient: A case report. 05-Apr-2024;15:123. Available from: https://surgicalneurologyint.com/surgicalint-articles/12840/
Abstract
Background: Spinal extradural arachnoid cysts comprise
Case Description: Here, we present a case of a 15-year-old female who presented with lower back pain radiating to her bilateral posterior thighs and knees, with imaging indicating a thoracolumbar spinal extradural arachnoid cyst. After failed conservative treatment, surgical intervention in the form of laminectomy, fenestration of the arachnoid cyst, and repair of the dural defect was required, resolving the patient’s symptoms with no recurrence of the cyst.
Conclusion: Complete resolution of pain in our patient following surgical management of spinal arachnoid cyst suggests that treatment of the arachnoid cyst can be achieved through minimal exposure to the site of the CSF leak to fenestrate the cyst and repair the leak.
Keywords: Case report, Dural defect, Laminectomy, Spinal arachnoid cyst
INTRODUCTION
Spinal arachnoid cysts are rare lesions of the spinal cord. They comprise only about 1–3% of all spinal masses.[
Spinal extradural arachnoid cysts (SEACs) consist of fluid-filled spaces within protrusions of arachnoid through a dural defect.[
Spinal extradural arachnoid cysts most commonly present within the 2nd–5th decades of life and are especially rare in the pediatric population.[
Here, we present a case of a 15-year-old female with symptoms of lower back pain, bilateral proximal leg pain and numbness as a result of spinal cord and nerve root compression by a spinal extradural arachnoid cyst.
CASE DESCRIPTION
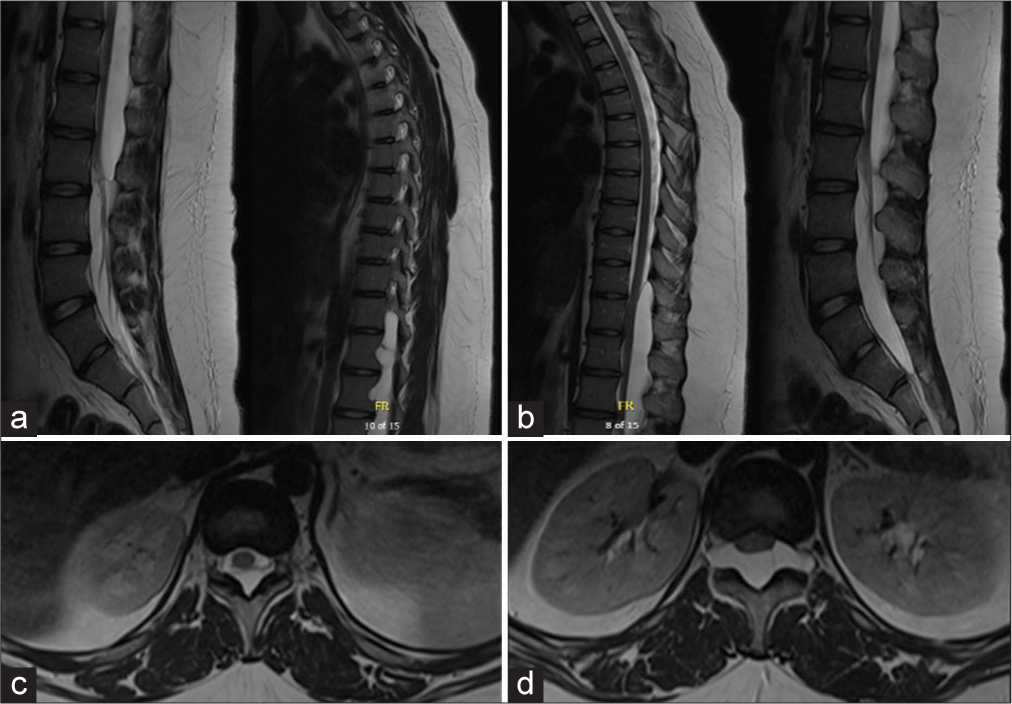
A 15-year-old female presented with three months of lower back pain radiating into her bilateral posterior thighs and knees. She had no history of trauma, infection, connective tissue disease, or previous spine condition. She had no weakness in her upper and lower extremities, bowel or bladder incontinence, saddle anesthesia, or difficulty ambulating. Twice daily ibuprofen and physical therapy for two months did not improve or resolve her symptoms. Thoracic and lumbar spine magnetic resonance imaging (MRI) demonstrated a large dorsal extradural meningeal cyst at T11–L2 with associated neural foraminal extension and bony expansion [
Instead of a traditional approach with multi-level laminectomy and given the identification of the likely site of the dural defect at L1, a minimally invasive approach in the form of L1–L2 laminectomy, fenestration of the arachnoid cyst, and L1 dural defect repair was selected to reduce the potential morbidity associated with a more extensive approach. Intraoperatively, the extradural cyst wall was located dorsally [
The patient had an uneventful postoperative period. Repeat imaging four months postoperatively demonstrated no residual extradural meningeal cyst [
DISCUSSION
Nabors et al. [
The pathogenesis of spinal arachnoid cysts is not well understood. Congenital dural defects are the most probable cause of SEAC, and identified small dural tears are often congenital in origin.[
SEACs are usually asymptomatic.[
The gold standard for diagnosis of SEAC is spinal MRI without contrast. Spinal MRI is useful for localizing the lesion, identifying the extent of the lesion, and demonstrating its relation to the dura and nerve root.[
Asymptomatic patients may not require treatment, and only long-term observation is indicated.[
Histological findings of resected SEAC walls do not have defining features for diagnosis. Characteristic findings include collagenous fibers or fibrous connective tissue with an inner single-cell lining.[
Our case presentation demonstrates the treatment of SEAC in a patient who presented only with symptoms of pain in the absence of other neurologic deficits. Conservative measures, including pain medication and physical therapy, failed to improve or resolve this patient’s symptoms. Rather, surgical fenestration of the cyst with dural repair resulted in a favorable outcome with the resolution of pain and no recurrence of the cyst. We propose treatment of SEAC with surgical management through this less invasive approach, even before the presentation of neurologic deficits, as opposed to waiting, mainly to provide this young patient with symptomatic relief. Previous studies have indicated that surgery in patients who have a more chronic course of SEAC results in less favorable outcomes and that earlier surgical repair demonstrates better clinical outcomes.[
CONCLUSION
Spinal arachnoid extradural cysts are rare lesions of the spinal cord that can result in motor, sensory, and bowel or bladder dysfunction due to spinal cord or nerve root compression. This case presents a rare finding in a pediatric patient with complete resolution of symptoms following cyst fenestration, partial resection of cyst wall, and dural repair of nerve root defect. Most cases are a result of congenital lesions and pressure changes in the subarachnoid space that drive fluid into the cystic cavity, which is the most accepted mechanism of cystic enlargement. Neuroimaging is diagnostic for spinal arachnoid cysts, and surgical management is standard for symptomatic treatment and prevention of recurrence. Histological findings demonstrate fibrous connective tissue with or without an inner single-cell arachnoid lining. We demonstrate the resolution of pain symptoms in our case presentation, suggesting that surgical intervention using our minimal approach should be pursued even in the absence of neurological deficit, resulting in the resolution of the cyst and improvement in symptoms.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Choi SW, Seong HY, Roh SW. Spinal extradural arachnoid cyst. J Korean Neurosurg Soc. 2013. 54: 355-8
2. De Oliveira F, Leira F, Braga L, Zamprogno P, Aversa A, Guimarães R. Extradural arachnoid cyst-case report and literature review. Interdiscip Neurosurg. 2021. 23: 100995
3. De Oliveira RS, Amato MC, Santos MV, Simão GN, Machado HR. Extradural arachnoid cysts in children. Childs Nerv Syst. 2007. 23: 1233-8
4. Eroglu U, Bozkurt M, Kahilogullari G, Dogan I, Ozgural O, Shah KJ. Surgical management of spinal arachnoid cysts in adults. World Neurosurgery. 2019. 122: e1146-52
5. Fam MD, Woodroffe RW, Helland L, Noeller J, Dahdaleh NS, Menezes AH. Spinal arachnoid cysts in adults: Diagnosis and management. A single-center experience. J Neurosurg Spine. 2018. 29: 711-9
6. Funao H, Nakamura M, Hosogane N, Watanabe K, Tsuji T, Ishii K. Surgical treatment of spinal extradural arachnoid cysts in the thoracolumbar spine. Neurosurgery. 2012. 71: 278-84
7. Lee CH, Hyun SJ, Kim KJ, Jahng TA, Kim HJ. What is a reasonable surgical procedure for spinal extradural arachnoid cysts: is cyst removal mandatory? Eight consecutive cases and a review of the literature. Acta Neurochir. 2012. 154: 1219-27
8. Lin L, Jason R. A rare case of spinal extradural arachnoid cyst with cord compression. Asian J Neurosurg. 2018. 13: 468-70
9. Liu JK, Cole CD, Kan P, Schmidt MH. Spinal extradural arachnoid cysts: Clinical, radiological, and surgical features. Neurosurg Focus. 2007. 22: E6
10. Nabors MW, Pait TG, Byrd EB, Karim NO, Davis DO, Kobrine AI. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988. 68: 366-77
11. Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T. Detection of a Dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine. 2004. 29: E426-30
12. Oh JK, Lee DY, Kim TY, Yi S, Ha Y, Kim KN. Thoracolumbar extradural arachnoid cysts: A study of 14 consecutive cases. Acta Neurochir. 2012. 154: 341-8
13. Oyemolade TA, Adeolu AA, Idowu OK. Spinal extradural arachnoid cyst in a child-a case report. J Surg Case Rep. 2019. 2019: rjz283
14. Payer M, Brühlhart K. Spinal extradural arachnoid cyst: Review of surgical techniques. J Clin Neurosci. 2011. 18: 559-60
15. Qi W, Zhao L, Fang J, Chang X, Xu Y. Clinical characteristics and treatment strategies for idiopathic spinal extradural arachnoid cysts: A single-center experience. Acta Neurochir. 2015. 157: 539-45
16. Ramazanoglu AF, Sarikaya C, Varol E, Aydin SO, Etli MU, Avci F. Surgical treatment of spinal arachnoid cysts: Cyst excision or fenestration?. Turk Neurosurg. 2022. 32: 1002-6
17. Sato K, Nagata K, Sugita Y. Spinal extradural meningeal cyst: Correct radiological and histopathological diagnosis. Neurosurg Focus. 2002. 13: ecp1
18. Satyarthee GD. Pediatric symptomatic sacral extradural arachnoid cyst: Surgical management review. J Pediatr Neurosci. 2018. 13: 211-3
19. Woo JB, Son DW, Kang KT, Lee JS, Song GS, Sung SK. Spinal extradural arachnoid cyst. Korean J Neurotrauma. 2016. 12: 185