- Chief of Neurosurgical Spine and Education, Department of Neurosurgery, Winthrop University Hospital, Mineola, New York – 11501, USA
Correspondence Address:
Nancy E. Epstein
Chief of Neurosurgical Spine and Education, Department of Neurosurgery, Winthrop University Hospital, Mineola, New York – 11501, USA
DOI:10.4103/2152-7806.191079
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Epstein NE. Extreme lateral lumbar interbody fusion: Do the cons outweigh the pros?. Surg Neurol Int 22-Sep-2016;7:
How to cite this URL: Epstein NE. Extreme lateral lumbar interbody fusion: Do the cons outweigh the pros?. Surg Neurol Int 22-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/extreme-lateral-lumbar-interbody-fusion-cons-outweigh-pros/
Abstract
Background:Major factors prompted the development of minimally invasive (MIS) extreme lateral interbody fusion (XLIF; NuVasive Inc., San Diego, CA, USE) for the thoracic/lumbar spine. These include providing interbody stabilization and indirect neural decompression while avoiding major visceral/vessel injury as seen with anterior lumbar interbody fusion (ALIF), and to avert trauma to paraspinal muscles/facet joints found with transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion (PLIF), and posterior-lateral fusion techniques (PLF). Although anticipated pros of MIS XLIF included reduced blood loss, operative time, and length of stay (LOS), they also included, higher fusion, and lower infection rates. Unanticipated cons, however, included increased morbidity/mortality rates.
Methods:We assessed the pros and cons (e.g., risks, complications, comparable value/superiority/inferiority, morbidity/mortality) of MIS XLIF vs. ALIF, TLIF, PLIF, and PLF.
Results:Pros of XLIF included various biomechanical and technical surgical advantages, along with multiple cons vs. ALIF, TLIF, PLIF, and PLF. For example, XLIF correlated with a considerably higher frequency of major neurological deficits vs. other constructs; plexus injuries 13.28%, sensory deficits 0–75% (permanent in 62.5%), motor deficits 0.7–33.6%, and anterior thigh pain 12.5–25%. XLIF also disproportionately contributed to other major morbidity/mortality; sympathectomy, major vascular injuries (some life-ending others life-threatening), bowel perforations, and seromas. Furthermore, multiple studies documented no superiority, and the potential inferiority of XLIF vs. ALIF, TLIF, PLIF, and PLF.
Conclusion:Reviewing the pros of XLIF (e.g. radiographic, technical, biomechanical) vs. the cons (inferiority, increased morbidity/mortality) vs. ALIF, TLIF, PLIF, and PLF, we question whether XLIF should remain part of the lumbar spinal surgical armamentarium.
Keywords: ALIF, comparison constructs, extreme lateral interbody fusion, lateral lumbar interbody fusion, minimally invasive surgery, posterior-lateral fusion techniques, posterior lumbar interbody fusion, transforaminal lumbar interbody fusion
INTRODUCTION
Minimally invasive surgery (MIS) consisting of extreme lateral interbody fusion procedures (XLIF) were devised to afford maximal disc excision and end plate availability for interbody fusion, while providing indirect decompression of the neural elements. Aims of MIS XLIF included avoiding the major visceral/vessel injuries seen with anterior lumbar interbody fusion (ALIF), and trauma to the posterior elements (e.g. paraspinal muscles/facet joints) seen with transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion (PLIF), and posterolateral fusion (PLF). Although further pros included the reduction of operative time, blood loss, length of stay (LOS), and duration of surgery, with potentially higher fusion and lower infection rates, there were also unanticipated cons of MIS XLIF included a disproportionate increase in the neurological/complications of spinal surgery vs. other constructs; i.e. plexus injuries 13.28%, sensory deficits 0–75% (permanent in 62.5%), motor deficits 0.7–33.6%, and anterior thigh pain 12.5–25%.[
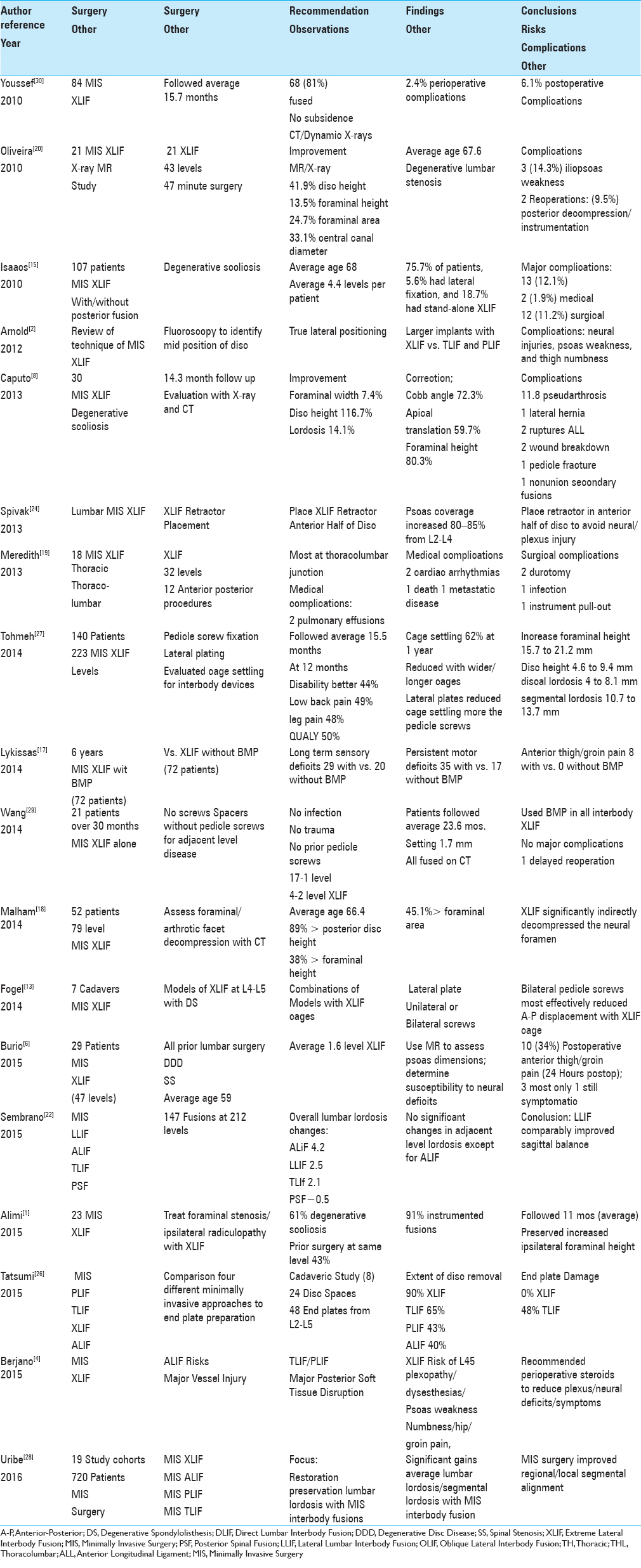
PROS AND CONS OF XLIF: X-RAY/COMPUTED TOMOGRAPHY (CT) AND BIOMECHANICS
Pros of X-ray/CT documentation of indirect decompression with extreme lateral interbody fusion
Postoperative X-rays and computed tomography (CT) studies documented that MIS XLIF with or without posterior instrumentation provided increased maximal disc removal/end plate availability for interbody fusion while affording indirect decompression of the spinal canal (degenerative stenosis or scoliosis) [
Biomechanical pros, cons, and comparability of minimally invasive surgeries (MIS) extreme lateral interbody fusion (XLIF)/lateral lumbar interbody fusion (LLIF) vs. other techniques
Several studies explored the biomechanical pros, cons, and comparability of MIS LLIF/XLIF vs. other procedures (e.g. ALIF, TLIF, PLIF, and PLF) (e.g., greater end plate/disc removal, restoration of sagittal balance and/or lordosis, but early cage settling) [
Pros of bilateral vs. unilateral pedicle screw fixation with minimally invasive surgery (MIS) extreme lateral interbody fusion (XLIF)
Several authors found that supplementing MIS XLIF with unilateral or bilateral pedicle screw fixation both increased lordosis, but bilateral instrumentation provided greater stabilization [Tables
Summary of computed tomography (CT)/X-rays and biomechanics minimally invasive surgery (MIS) extreme lateral interbody fusion (XLIF) pros/cons
Pros for MIS XLIF vs. ALIF, TLIF, PLIF, and PLF included more disc removal/end plate availability for interbody fusion, and greater indirect neural decompression by increasing disc height/foraminal height/area/canal diameter.[
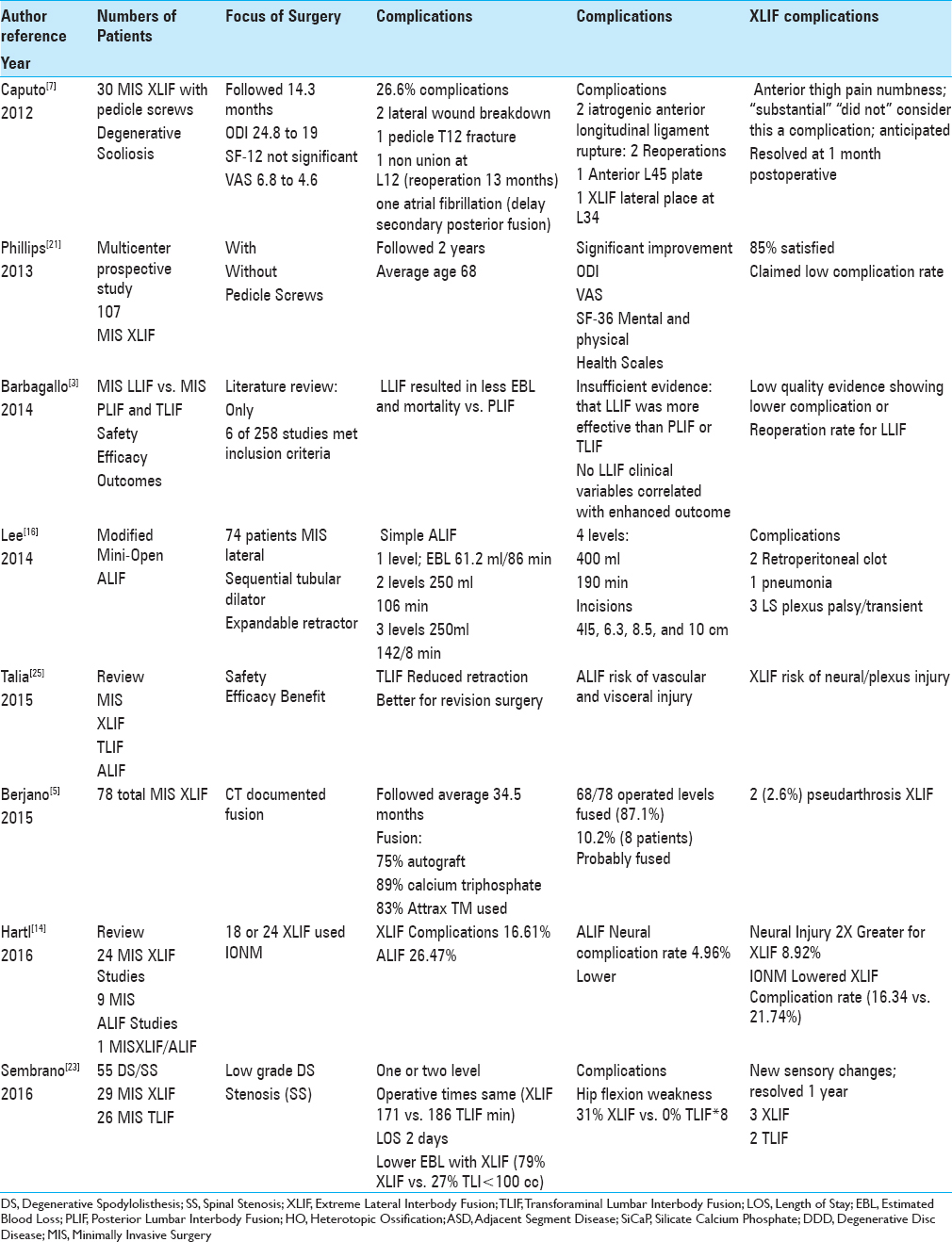
CONS OF MINIMALLY INVASIVE SURGERY EXTREME LATERAL INTERBODY FUSION
High complication rate for minimally invasive surgery surgery (MIS) extreme lateral interbody fusion (XLIF) vs. Other minimally invasive surgery constructs (ALIF, TLIF, PLIF, PLF)
Neurological complications of extreme lateral interbody fusion vs. other procedures
Neurological complications frequently followed thoracic and lumbar MIS XLIF vs. other constructs that some preferred to label as “anticipated” risks rather than “complications” [Tables
Cadaver and magnetic resonance/dynamically-evoked electromyography offer technical improvements for minimally invasive surgery extreme lateral interbody fusion procedures, but neurological deficits persist
Two studies, one performed in cadavers and the other performed utilizing magnetic resonsnce images (MR), sought to limit the common MIS XLIF postoperative lumbar plexus deficits [
Fluoroscopy and computed tomography (CT) studies offer technical improvements for minimally invasive surgery (MIS) extreme lateral interbody fusion (XLIF) procedures, but neurological deficits still persist
Utilizing intraoperative X-ray/fluoroscopy in combination with postoperative CT examinations helped guide the performance of thoracic and lumbar MIS XLIF procedures [
Neurological complications of minimally invasive surgery surgery (MIS) extreme lateral interbody fusion (XLIF) with bone morphogenetic protein: Reported vs. “obfuscated” results
Additional unique complications occurred when bone morphogenetic protein (rhBMP-2) was utilized to supplement MIS XLIF/LLIF constructs [Tables
Non-neurological complications of minimally invasive surgery surgery (MIS) extreme lateral interbody fusion (XLIF) vs. other procedures
Multiple additional medical/surgical complications, excluding neurological deficits, were attributed to MIS XLIF [Tables
Lack of safety, efficacy, and superiority (some say inferiority) of minimally invasive surgery (MIS) extreme lateral interbody fusion (XLIF) over other constructs
Multiple studies demonstrated a lack of safety or efficacy of MIS XLIF over other available fusion constructs (e.g. MIS, ALIF, TLIF, PLIF, and PLF) (e.g. particularly regarding perioperative neurological/other morbidity) [
Lack of superiority and potential inferiority of minimally invasive surgery surgery (MIS) extreme lateral interbody fusion (XLIF) vs. other constructs
Multiple studies emphasized either the lack of superiority of MIS XLIF over other constructs or in some cases, MIS XLIF's lesser performance [Tables
CONCLUSION
MIS XLIF were originally devised to provide increased end plate availability for interbody spinal fusion to better facilitate arthrodesis rates while providing indirect neural decompression. Anticipated major advantages included avoiding major vessel/visceral injuries seen with MIS ALIF, trauma to the posterior elements, and reduced operative time, blood loss, LOS vs. MIS TLIF/PLIF and PLF. Nevertheless, these multiple studies failed to document the safety, efficacy, or superiority of the MIS XLIF vs. the multiple other surgical alternatives. In fact, they documented the increased neurological and surgical/medical complication rates for XLIF that were in some instances life-threatening, or even, life-ending.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alimi M, Hofstetter CP, Tsiouris AJ, Elowitz E, Härtl R. Extreme lateral interbody fusion for unilateral symptomatic vertical foraminal stenosis. Eur Spine J. 2014. 24: 346-52
2. Arnold PM, Anderson KK, McGuire RA. The lateral transpsoas approach to the lumbar and thoracic spine: A review. Surg Neurol Int. 2012. 3: S198-215
3. Barbagallo GM, Albanese V, Raich AL, Dettori JR, Sherry N, Balsano M. Lumbar Lateral Interbody Fusion (LLIF): Comparative Effectiveness and Safety versus PLIF/TLIF and Predictive Factors Affecting LLIF Outcome. Evid Based Spine Care J. 2014. 5: 28-37
4. Berjano P, Gautschi OP, Schils F, Tessitore E. Extreme lateral interbody fusion (XLFI): How I do it. Aca Neurochir. 2015. 157: 547-51
5. Berjano P, Langella , Damilano M, Pejrona M, Buric J, Ismael M. Fusion rate following extreme lateral lumbar interbody fusion. Eur Spine J. 2015. 24: 369-71
6. Buric J. Relationship between psoas muscle dimensions and postoperative thigh pain. A possible preoperative evaluation factor. Int J Spine Surg. 2015. 9: 27-
7. Caputo AM, Michael KW, Chapman TM, Massey GM, Howes CR, Isaacs RE. Clinical outcomes of extreme lateral interbody fusion in the treatment of adult degenerative scoliosis. ScientificWorldJournal 2012. 2012. p. 680643-
8. Caputo AM, Michael KW, Chapman TM, Jennings JM, Hubbard EW, Isaacs RE. Extreme lateral interbody fusion for the treatment of adult degenerative scoliosis. J Clin Neurosci. 2013. 20: 1558-63
9. Epstein NE. More nerve root injuries occur with minimally invasive lumbar surgery, especially extreme lateral interbody fusion: A review. Surg Neurol Int. 2016. 7: S83-95
10. Epstein NE. More nerve root injuries occur with minimally invasive lumbar surgery: Let's tell someone. Surg Neurol Int. 2016. 7: S96-S101
11. Epstein NE. High Neurological Complication Rates for Extreme Lateral Lumbar Interbody Fusion (XLIF) and Related Techniques: A Review of Safety Concerns. Surgical Neurol Int. 2016. p.
12. Epstein NE. Editorial Non-Neurological Complications of Extreme Lateral Lumbar Interbody Fusion Techniques (XLIF) and Related Procedures. Surg Neurol Int. 2016. p.
13. Fogel GR, Turner AW, Dooley ZA, Cornwall GB. Biomechanical stability of lateral interbody implants and supplemental fixation in a cadaveric degenerative spondylolisthesis model. Spine. 2014. 39: E1138-46
14. Härtl R, Joeris A, McGuire RA. Comparison of the safety outcomes between two surgical approaches for anterior lumbar fusion surgery: Anterior lumbar interbody fusion (ALIF) and extreme lateral interbody fusion (ELIF). Eur Spine J. 2016. 25: 1484-521
15. Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: Perioperative outcomes and complications. Spine. 2010. 35: S322-30
16. Lee CS, Chung SS, Pae YR, Park SJ. Mini-open approach for direct lateral lumbar interbody fusion. Asian Spine J. 2014. 8: 491-7
17. Lykissas MG, Aichmair A, Sama AA, Hughes AP, Lebl DR, Cammisa FP. Nerve injury and recovery after lateral lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: A cohort-controlled study. Spine J. 2014. 14: 217-24
18. Malham GM, Parker RM, Goss B, Blecher CM, Ballok ZE. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion. Spine. 2014. 39: E1303-10
19. Meredith DS, Kepler CK, Huang RC, Hegde VV. Extreme Lateral Interbody Fusion (XLIF) in the Thoracic and Thoracolumbar Spine: Technical Report and Early Outcomes. HSS J. 2013. 9: 25-31
20. Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine. 2010. 35: S331-7
21. Phillips FM, Isaacs RE, Rodgers WB, Khajavi K, Tohmeh AG, Deviren V. Adult degenerative scoliosis treated with XLIF: Clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine. 2013. 38: 1853-61
22. Sembrano JN, Yson SC, Horazdovsky RD, Santos ER, Polly DW. Radiographic Comparison of Lateral Lumbar Interbody Fusion Versus Traditional Fusion Approaches: Analysis of Sagittal Contour Change. Int J Spine Surg. 2015. 9: 16-
23. Sembrano JN, Tohmeh A, Isaacs R. Two-year Comparative Outcomes of MIS Lateral and MIS Transforaminal Interbody Fusion in the Treatment of Degenerative Spondylolisthesis. Part I: Clinical Findings. Spine. 2016. 41: S123-32
24. Spivak JM, Paulino CB, Patel A, Shanti N, Pathare N. Safe zone for retractor placement to the lumbar spine via the transpsoas approach. J Orthop Surg. 2013. 21: 77-81
25. Talia AJ, Wong ML, Lau HC, Kaye AH. Comparison of the different surgical approaches for lumbar interbody fusion. J Clin Neurosci. 2015. 22: 243-51
26. Tatsumi R, Lee YP, Khajavi K, Taylor W, Chen F, Bae H. In vitro comparison of endplate preparation between four mini-open interbody fusion approaches. Eur Spine J. 2015. 24: 372-7
27. Tohmeh AG, Khorsand D, Watson B, Zielinski X. Radiographical and clinical evaluation of extreme lateral interbody fusion: Effects of cage size and instrumentation type with a minimum of 1-year follow-up. Spine. 2014. 39: E1582-91
28. Uribe JS, Myhre SL, Youssef JA. Preservation or Restoration of Segmental and Regional Spinal Lordosis Using Minimally Invasive Interbody Fusion Techniques in Degenerative Lumbar Conditions: A Literature Review. Spine. 2016. 41: S50-8
29. Wang MY, Vasudevan R, Mindea SA. Minimally invasive lateral interbody fusion for the treatment of rostral adjacent-segment lumbar degenerative stenosis without supplemental pedicle screw fixation. J Neurosurg Spine. 2014. 21: 861-6
30. Youssef JA, McAfee PC, Patty CA, Raley E, DeBauche S, Shucosky E. Minimally invasive surgery: lateral approach interbody fusion: Results and review. Spine. 2010. 35: S302-11