- Department of Neurosurgery, Army Hospital Research and Referral, Delhi,
- Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh,
- Department of Neurosurgery, All India Institute of Medical Sciences, Delhi,
- Department of Anaesthesia and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Raj Ratan, Department of Neurosurgery, Army Hospital Research and Referral, Delhi, India.
DOI:10.25259/SNI_754_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Raj Ratan1, Sandeep Mohindra2, Manjul Tripathi2, Raghav Singla3, Rajeev Chauhan4. Factors determining the requirement of surgical intervention and prognosis in cases of traumatic bifrontal contusions: A prospective observational study. 22-Dec-2023;14:438
How to cite this URL: Raj Ratan1, Sandeep Mohindra2, Manjul Tripathi2, Raghav Singla3, Rajeev Chauhan4. Factors determining the requirement of surgical intervention and prognosis in cases of traumatic bifrontal contusions: A prospective observational study. 22-Dec-2023;14:438. Available from: https://surgicalneurologyint.com/surgicalint-articles/12683/
Abstract
Background: Traumatic brain injury, being a notorious cause of mortality and morbidity across the globe, presents with a variety of lesions. One of the distinct patterns of injury is characterized by contusions of both frontal lobes, labeled “traumatic bifrontal contusions” (TBCs). TBC is often associated with the presence of significant edema and mass effect leading to rapid clinical deterioration after a usually benign presentation at the time of first evaluation. Formulating a management plan in a patient with TBC is often more difficult than in a patient with a major intracranial hematoma.
Methods: A prospective observational study with aims and objectives to identify predictors of an unfavorable outcome, analysis of the evolution of TBC, evaluation of the specific indications for surgery, and determination of the prognosis. All head trauma patients harboring bifrontal contusions were included in the study. Patients with other associated operable injuries involving blunt trauma abdomen and orthopedic injuries, counter-coupe injuries, and obvious open fractures noted over calvaria were excluded from the study. Glasgow coma scale (GCS) was recorded during the first assessment, followed by non-contrast computerized tomography (NCCT) Head.
Results: A total of 53 patients satisfying inclusion and exclusion criteria were included in the study. The average GCS score recorded before surgical intervention was 9. The mean and median best motor response noted was M5. The interval from the time of injury to the first NCCT of the brain at the study hospital ranged from 3 h to 163 h, averaging 17.66 h. The median category w.r.t Marshall’s CT classification observed was “Diffuse Injury IV.” The volume of the contusions in each scan was estimated, and the average anterior cranial fossa volume observed was 125 mL. “Upfront surgery” (“Bifrontal decompressive craniectomy” or “unilateral Fronto-Temporo-Parietal [FTP] decompressive hemicraniectomy”) was carried out on the day of admission based on the findings on the first NCCT brain. About 49% of patients at presentation needed surgical intervention as per existing protocols. The duration of observation for patients who were initially observed but eventually had to undergo surgery ranged from 1 to 5 days, with an average observation period of 2 days. The duration of observation in those who did not subsequently need surgery ranged from 7 to 10 days, with an average duration of 9 days.
Conclusion: What leads to the poorly predictable, delayed, and rapid deterioration that sets TBCs apart from other traumatic brain injuries is still unclear. Our study finds that having a low threshold for repeat CT imaging of the patient led to earlier identification of progression, and a low threshold for surgical intervention led to favorable outcomes.
Keywords: Traumatic bifrontal contusion, Upfront surgery, Rapid clinical deterioration
INTRODUCTION
Traumatic brain injury (TBI), being a notorious cause of mortality and morbidity across the globe, presents with a variety of lesions. Traumatic damage to brain parenchyma in the form of contusions has been seen with increasing frequency.[
MATERIALS AND METHODS
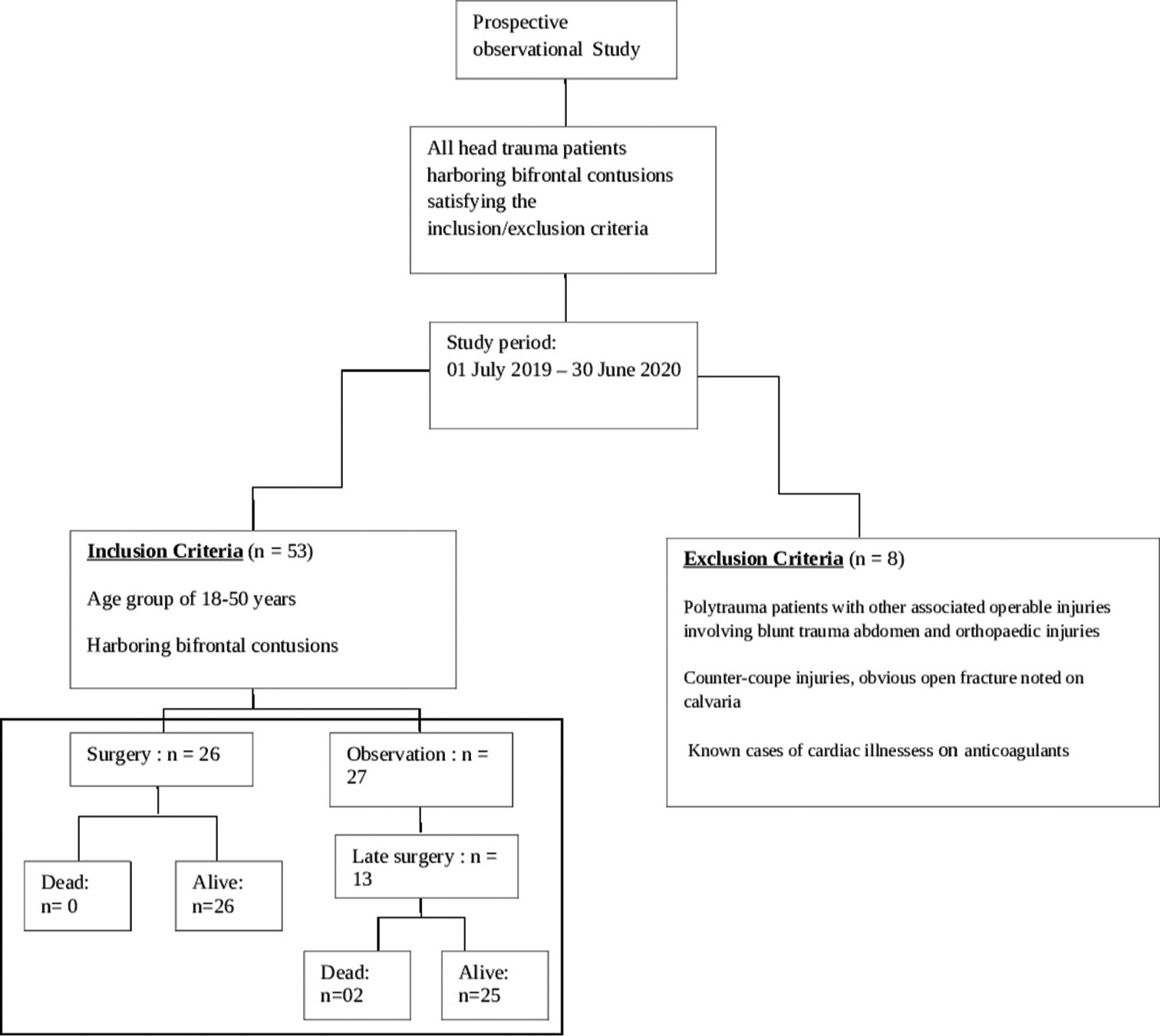
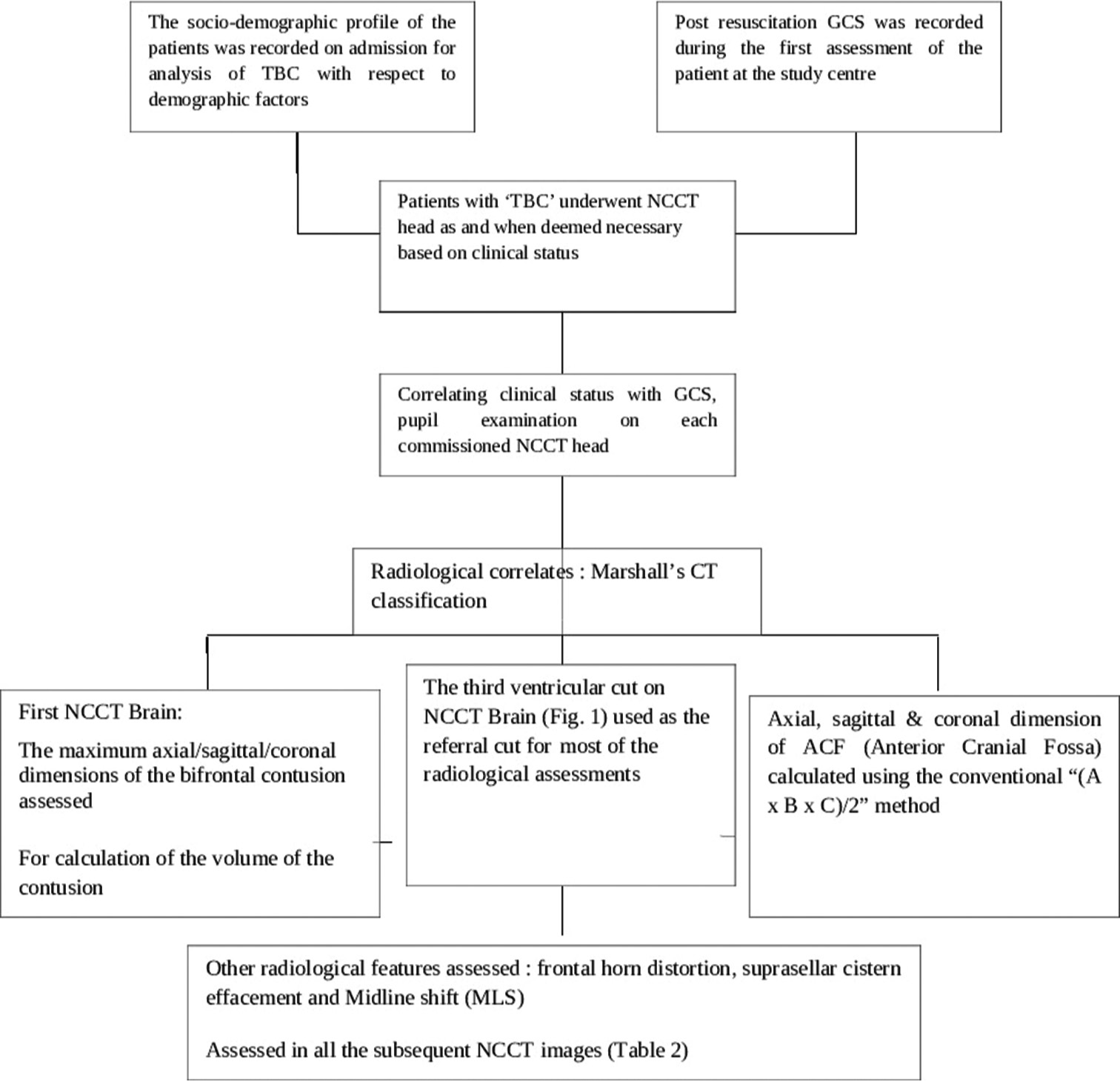
The study has been approved by the Institutional Ethical Committee vide reference no INT/IEC/2020/SPL-1466 dt 18 Nov 2020. The study design and protocol have been elaborated in the flowchart in
RESULTS
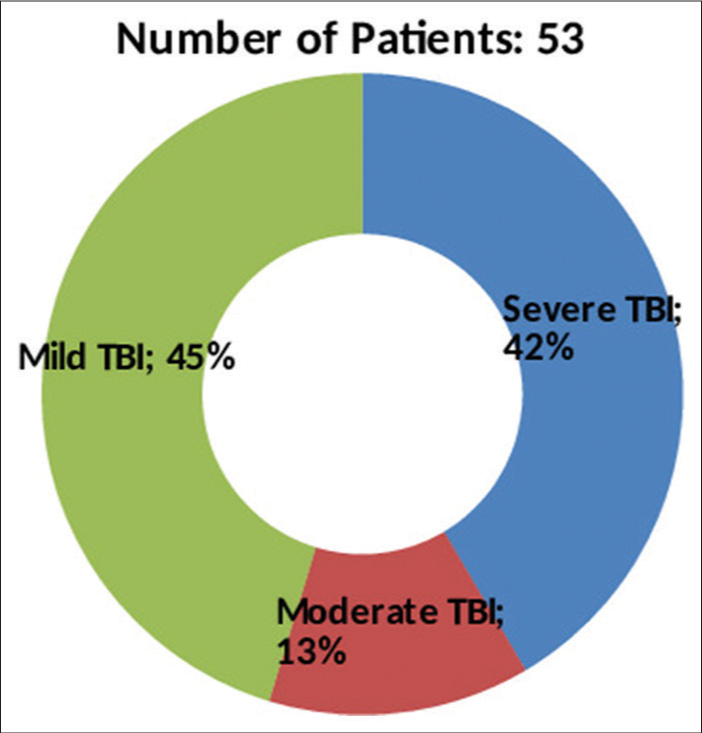
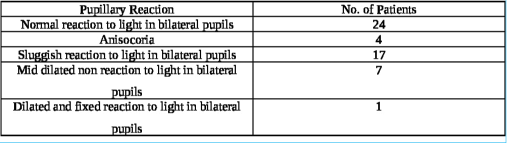
A total of 53 patients satisfying inclusion and exclusion criteria were included in the study. The study population consisted of 48 (90.6%) males and 05 (9.4%) females with ages ranging from 18 to 50 years (average age 37.2 years). The causes of TBI were road traffic accidents (RTA) in 38 (71.7%), fall from height in 12 (22.6%), assault in 2, and other mode of injury in one patient. The initial post-resuscitation Glasgow coma scale (GCS) score ranged from 4 to 15 and averaged 10. The mean and median best motor response noted was M5. The severity of TBI across the patient population according to admission GCS is depicted in
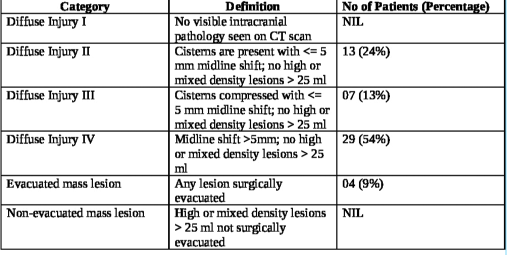
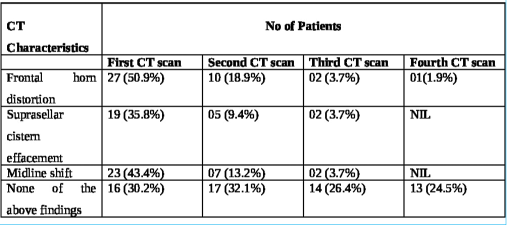
The interval from the time of injury to the first non-contrast computerized tomography (NCCT) of the brain at the study hospital ranged from 3 h to 163 h, averaging 17.66 h. The longest interval for the first NCCT Head was 163 h in one patient as the patient could not be attended at a medical establishment with CT scan facilities before being attended at the study hospital. Marshall’s CT classification for TBI was utilized for radiological characterization of the bifrontal contusions, and the category-wise distribution in the study population is noted in
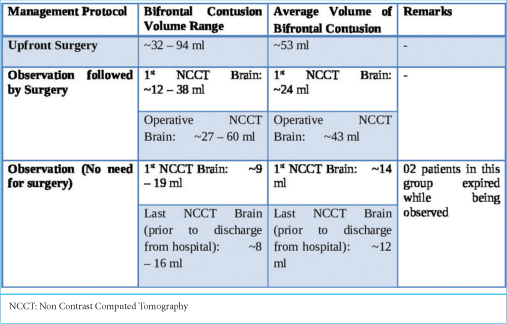
The approximate volume of anterior cranial fossa (ACF) estimated in the study population ranged from 110 mL to 140 mL, with an average volume of 125 mL among the 53 patients. The volume of the contusions in each scan was estimated.
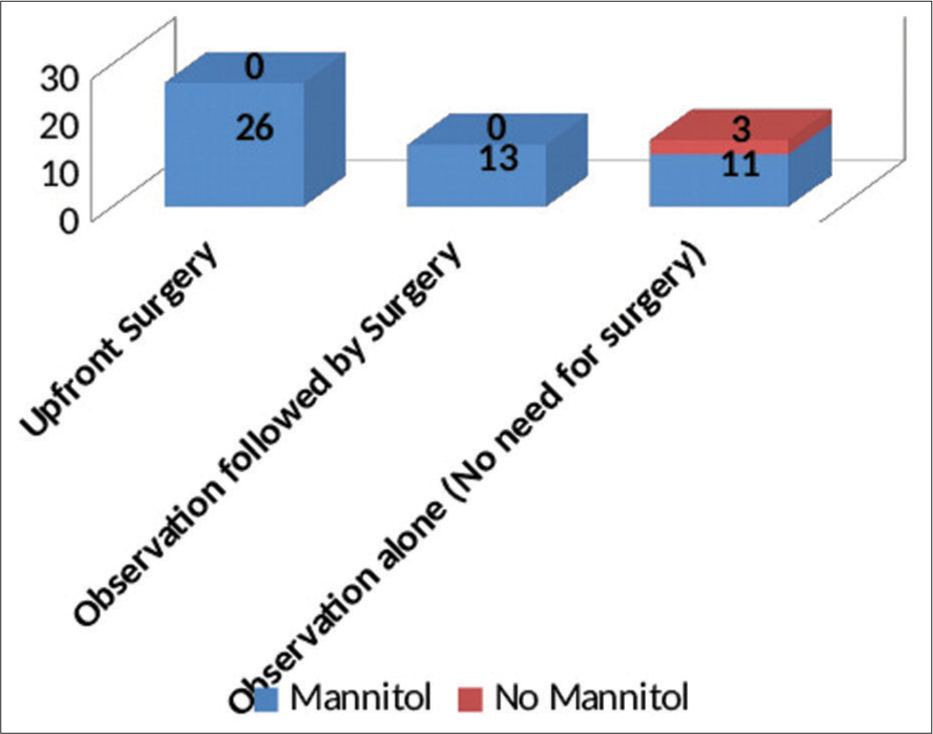
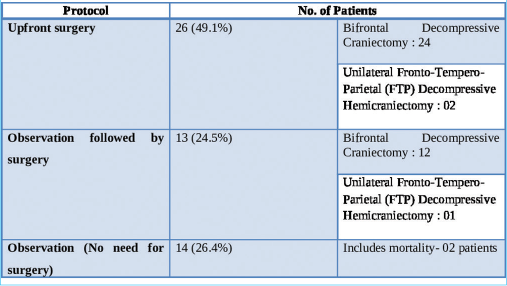
With regard to the management, the study population has been divided into three distinct groups:
Upfront surgery comprising of either “Bifrontal decompressive craniectomy” or “unilateral FTP decompressive hemicraniectomy” Observation followed by surgery (“Bifrontal decompressive craniectomy” or “unilateral FTP decompressive hemicraniectomy”) Observation alone (No surgery done).
“Upfront surgery” was carried out on the day of admission based on the findings on the first NCCT brain. About 49% of patients at presentation needed surgical intervention as per existing protocols. The criteria for surgery were as follows:-
The presence of large-volume bifrontal contusions with the findings of poor GCS Presence of frontal horn distortion, suprasellar cistern effacement, and significant mass effect.
These patients underwent decompressive craniectomy with or without evacuation of hematomas/contusions. In most patients undergoing contusectomy, falx was not divided. Two patients who would otherwise have been candidates for upfront surgical decompression were in an unfit state for surgery and succumbed to their injuries. In the rest, a CT of the brain was done on each day following admission so as to assess for radiological deterioration. Medical management involved hyperosmolar therapy with mannitol in patients who demonstrated mass effect on imaging while being neurologically as well as hemodynamically stable [
The duration of observation for patients who were initially observed but eventually had to undergo surgery ranged from 1 to 5 days, with an average observation period of 2 days. The duration of observation in those who did not subsequently need surgery ranged from 7 to 10 days, with an average duration of 9 days.
DISCUSSION
TBC is a common cerebral injury that may not appear very serious at the outset but has a tendency to evolve in a poorly predictable manner and abruptly turn life-threatening if not managed in a timely fashion. The primary finding of the present study is that the clinicoradiological course of patients with isolated TBC varies from that described in the literature for traumatic cerebral contusions elsewhere in the brain. Contusions, rather than extra-axial ICHs, have been increasingly known to occur in recent times, with rising numbers of low-velocity injuries in the developed world.[
To do or not to do: Early versus delayed deterioration
The first challenge of decision-making was surmounted fairly easily in the “upfront surgery” group. The remainder of the patients admitted for observation with relatively better initial clinical and radiological pictures (i.e., nearly half the study population) mandated the development of a foolproof algorithm for management. The rate of clinical deterioration seen in our study population stands in contrast to existing worldwide literature studying the progression of traumatic cerebral contusions in certain pertinent aspects. A fourth (24.5%) of our TBC patients admitted for observation went on to require surgery. Nine out of these 13 patients deteriorated within 48 h of admission and underwent surgery. Alahmadi et al.,[
Blossoming of contusions: An unknown phenomenon
An important factor pointed out in the literature that contributes to significant progression is contusion size. Contusion size at presentation, in itself, appears to determine immediate management. Attempts have been made to delineate a cutoff size below which contusions may not require surgery. The mean contusion volume at which patients were taken for upfront decompressive craniectomy in our study was 43 mL. The ratio between volumes of bifrontal contusion to volume of ACF ranged from 0.067 to 0.721, with a mean ratio of 0.289. The smallest bifrontal contusion was 6.7% of the volume of ACF, and the largest bifrontal contusion involved 72.1% of the volume of ACF. Associated intracranial injuries, besides the contused frontal brain itself, also play a very significant role in determining the management path. In the study by Peterson and Chesnut [
The average GCS at presentation in our series was 10. Over 45% of patients presented with mild head injury (GCS ≥ 13), and of these, 54% of patients deteriorated within 48 h of admission and had to undergo decompressive craniectomy. The distribution of patients across the categories of mild, moderate, and severe head injury was similar to the study populations of other series, including Zhaofeng et al.[
Predictive markers of deterioration
It is important to consider the two aspects of deterioration in these traumatic lesions: clinical deterioration and radiographic progression. No specific study has ever analyzed the link or difference in timing between the two. Peterson and Chesnut[
An important objective of this study was to evaluate specific indications for surgical intervention. Adequate analgesia, sedation, institution of hyperosmolar therapy, and correction of coagulation dysfunction must be immediately undertaken on admission. In cases where surgery is indicated, a decompressive craniectomy is the most useful among available methods to control ICP. The selection of the surgical time is critical, considering the rapid development of central brain herniation and the poor prognosis associated with it. The fate of the patient is decided by the duration for which the effects of central herniation persist. It is apparent in our study that early surgical intervention has resulted in a good prognosis for patients. Brain Trauma Foundation guidelines[
CONCLUSION
What leads to the poorly predictable, delayed, and rapid deterioration that sets TBCs apart from other traumatic brain injuries is still unclear. During the admission period, even a small drop in GCS (1–2 points) is of paramount importance in these cases. Our study finds that having a low threshold for repeat CT imaging of the patient led to earlier identification of progression, and a low threshold for surgical intervention led to a favorable outcome. Further studies are needed to develop a uniform, systematic approach to patients with TBCs.
Ethical approval
The author(s) declare that they have taken the ethical approval from IEC vide reference no INT/IEC/2020/SPL-1466 dated 18 November 2020.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adatia K, Newcombe VF, Menon DK. Contusion progression following traumatic brain injury: A review of clinical and radiological predictors, and influence on outcome. Neurocrit Care. 2021. 34: 312-24
2. Alahmadi H, Vachhrajani S, Cusimano MD. The natural history of brain contusion: An analysis of radiological and clinical progression. J Neurosurg. 2010. 112: 1139-45
3. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeo. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007. 24: S1-106
4. Chang EF, Meeker M, Holland MC. Acute traumatic intraparenchymal hemorrhage: Risk factors for progression in the early post-injury period. Neurosurgery. 2006. 58: 647-56
5. Gao L, Wu X, Hu J, Jin Y, Han X, Wu X. Intensive management and prognosis of 127 cases with traumatic bilateral frontal contusions. World Neurosurg. 2013. 80: 879-88
6. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008. 7: 728-41
7. Narayan RK, Maas AI, Servadei F, Skolnick BE, Tillinger MN, Marshall LF. Progression of traumatic intracerebral hemorrhage: A prospective observational study. J Neurotrauma. 2008. 25: 629-39
8. Peterson EC, Chesnut RM. Talk and die revisited: bifrontal contusions and late deterioration. J Trauma. 2011. 71: 1588-92
9. Sarma PS, Shukla DP, Devi BI. Bifrontal contusions: What is the best surgical treatment?. Indian J Neurotrauma. 2015. p.
10. Statham PF, Johnston RA, Macpherson P. Delayed deterioration in patients with traumatic frontal contusions. J Neurol Neurosurg Psychiatry. 1989. 52: 351-4
11. Van de Zande N, Manivannan S, Sharouf F, Shastin D, Abdulla M, Chumas PD. Demographics, presentation, and clinical outcomes after traumatic bifrontal contusions: A systematic review. Neurosurg Rev. 2020. 43: 977-86
12. Wang H, Xin T, Sun X, Wang S, Guo H, Holton-Burke C. Posttraumatic seizures--a prospective, multicenter, large case study after head injury in China. Epilepsy Res. 2013. 107: 272-8
13. Zhaofeng L, Bing L, Peng Q, Jiyao J. Surgical treatment of traumatic bifrontal contusions: When and how?. World Neurosurg. 2016. 93: 261-9