- Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
Correspondence Address:
Ha Son Nguyen
Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
DOI:10.4103/2152-7806.175899
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nguyen HS, Doan N, Wolfla C, Pollock G. Fenestration of bone flap during decompressive craniotomy for subdural hematoma. Surg Neurol Int 08-Feb-2016;7:16
How to cite this URL: Nguyen HS, Doan N, Wolfla C, Pollock G. Fenestration of bone flap during decompressive craniotomy for subdural hematoma. Surg Neurol Int 08-Feb-2016;7:16. Available from: http://surgicalneurologyint.com/surgicalint_articles/fenestration-of-bone-flap-during-decompressive-craniotomy-for-subdural-hematoma/
Abstract
Background:Persistent/recurrent extra-axial hemorrhage may occur after decompression of a subdural hematoma (SDH) followed by an immediate replacement of bone flap. A fenestration of the bone flap may encourage extra-axial fluid absorption; however, the literature has not explored this technique.
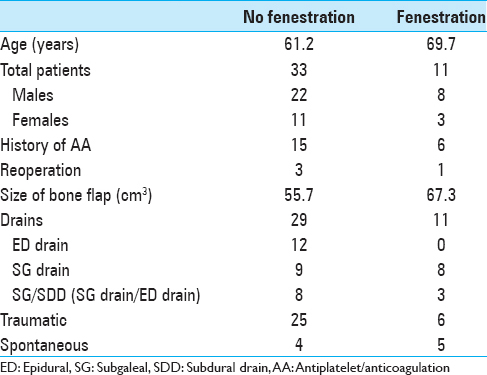
Methods:Forty-four consecutive patients who underwent surgical decompression of SDH with immediate replacement of bone flap were divided into two groups: Fenestration (F), n = 33, and no fenestration (NF), n = 11. Fenestration involves placement of twist drill holes 1–2 cm apart throughout the bone flap. Clinical data (age, sex, history of antiplatelet/anticoagulation [AA], and presence of drains) were collected. The size of bone flap, postoperative volume, and midline shift (MLS) were measured. A univariate analysis was performed for continuous variables; Fisher's exact test was performed for categorical variables.
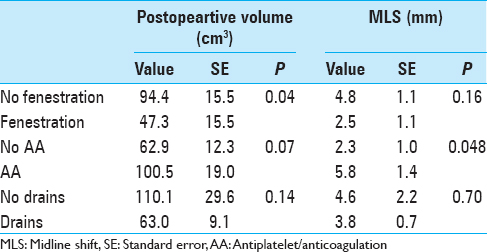
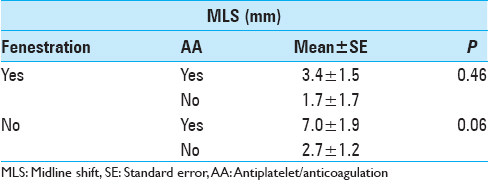
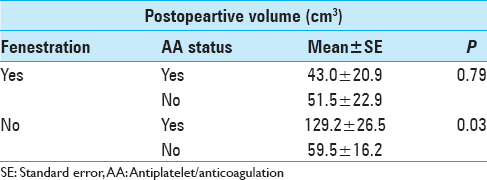
Results:For postoperative volume, NF group exhibited 94.4 ± 15.5 cm3, while F group exhibited 47.3 ± 15.5 cm3 (P = 0.04); no AA exhibited 62.9 ± 12.3 cm3, while AA exhibited 100.5 ± 19.0 cm3 (P = 0.07); no drains exhibited 110.1 ± 29.6 cm3, while drains exhibited 63.0 ± 9.1 cm3 (P = 0.14). For postoperative MLS, NF group exhibited 4.8 ± 1.1 mm, while F group exhibited 2.5 ± 1.1 mm (P = 0.16); no AA exhibited 2.3 ± 1.0 mm, while AA exhibited 5.8 ± 1.4 mm (P = 0.048); no drains exhibited 4.6 ± 2.2 mm, while drains exhibited 3.8 ± 0.7 mm (P = 0.70). Accounting for fenestration status and AA status: For F group, AA status did not correlate with postoperative volume or MLS significantly; for NF group, history of AA exhibited higher postoperative value 129.2 ± 26.5 cm3, compared to no history of AA at 59.5 ± 16.2 cm3 (P = 0.03).
Conclusion:Our results suggest that fenestration prior to the immediate replacement of bone flap after surgical decompression of SDH has the potential to reduce extra-axial fluid accumulation.
Keywords: Bone flap fenestration, reoperation, subdural hematoma
INTRODUCTION
The decision for an immediate replacement of the bone flap after surgical decompression of a subdural hematoma (SDH) is typically based on the personal experience of the operating surgeon. A significant concern after immediate replacement is a higher potential for intracranial hypertension, attributable to cerebral edema, contusions, and persistent intracranial hemorrhage and/or recurrence of hemorrhage. The latter may require reoperation, with reported incidences ranging up to 6–16%.[
METHODS
The approval of the institutional board review at our hospital was obtained prior to the study.
Between fall 2012 and spring 2015, 44 patients underwent surgical decompression for SDH with immediate replacement of the bone flap at our institution. A standard fronto-temporo-parietal craniotomy is performed, and the dura is opened in a stellate fashion. Once the hematoma is evacuated and hemostasis is achieved, the dural leaves of the stellate opening are loosely reflected back over the brain and a sizeable layer of nonsuturable Duragen is applied to cover the entire dural opening. No attempt is made to create a watertight duraplasty. A drain is frequently placed superficial to the Duragen and is referred to as an epidural (ED) drain. In addition, a SG drain may also be placed.
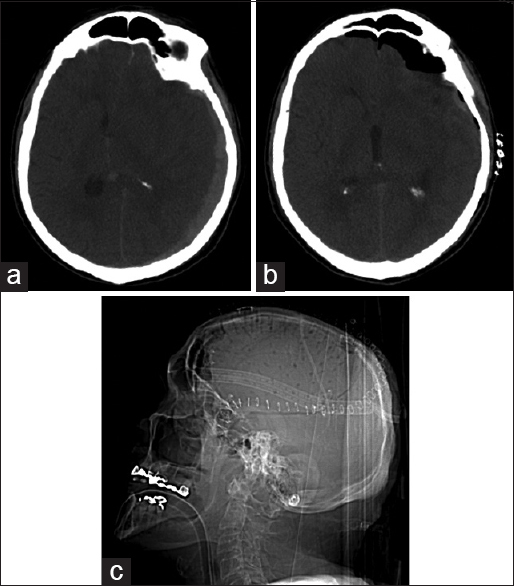
Eight neurosurgeons performed the surgeries. The patients were divided into two groups: Fenestration (F) and no fenestration (NF). The fenestration involves placement of twist drill holes 1–2 cm apart throughout the bone flap [Figure
Data were entered into SPSS version 22 (IBM Corporation, Armonk, NY, USA) for analysis. Results were expressed as the means ± standard error for all descriptive continuous variables. A univariate analysis and Fisher's exact test were performed for continuous variables and categorical variables respectively. A P < 0.05 was considered statistically significant.
RESULTS
DISCUSSION
DC for SDH, where the bone flap is replaced after surgical decompression, excludes the need for an interval cranioplasty. In particular, an additional procedure puts patients at risk for complications (hydrocephalus, infection, seizures, and hemorrhage). However, a risk after immediate bone flap replacement is a higher potential for intracranial hypertension, attributable to cerebral edema, contusions, and persistent/recurrent extra-axial hemorrhage. These factors may require reoperation with reported incidences ranging up to 6–16%.[
To our knowledge, the modification for fenestration of bone flap during DC has not been reported. On the other hand, fenestration has been employed with synthetic implants for interval cranioplasty, including polyether ether ketone implants and titanium mesh; unfortunately, limited data exist with respect to extra-axial fluid accumulation and subsequent reoperation.[
Several studies have evaluated the influence of oral antithrombotic therapy during the management of acute SDH. Panczykowski et al.[
The contribution of drains during DC has not been widely explored. Data regarding the use of drains during interval cranioplasty is conflicting.[
The limitations of this study include the retrospective analysis, entailing possible selection bias and loss of patient data. Moreover, the study was purely a radiological study, where outcome data (including mortality, morbidity, Glasgow scale outcome scores) were not evaluated. Moreover, operative techniques were varied among surgeons. On the other hand, the patients in the two groups appeared comparable, given that the age and bone flap size were not significantly different.
CONCLUSION
Our results suggest that fenestration prior to immediate replacement of bone flap after surgical decompression of SDH has the potential to reduce extra-axial fluid accumulation. To our knowledge, the modification for fenestration of bone flap during DC has not been reported. Theoretically, the fenestrations provide a more surface area for drainage of extra-axial fluid through the bone flap. Moreover, history of AA therapy may increase the risk of postoperative fluid accumulation. Randomized control studies should evaluate this technique as a modification to DC procedure. This technique may help moderate intracranial hypertension.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adeleye AO, Azeez AL. Decompressive craniectomy bone flap hinged on the temporalis muscle: A new inexpensive use for an old neurosurgical technique. Surg Neurol Int. 2011. 2: 150-
2. Bershad EM, Farhadi S, Suri MF, Feen ES, Hernandez OH, Selman WR. Coagulopathy and inhospital deaths in patients with acute subdural hematoma. J Neurosurg. 2008. 109: 664-9
3. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
4. Kenning TJ, Gandhi RH, German JW. A comparison of hinge craniotomy and decompressive craniectomy for the treatment of malignant intracranial hypertension: Early clinical and radiographic analysis. Neurosurg Focus. 2009. 26: E6-
5. Kenning TJ, Gooch MR, Gandhi RH, Shaikh MP, Boulos AS, German JW. Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: Decompressive craniectomy and hinge craniotomy. J Neurosurg. 2012. 116: 1289-98
6. Klinger DR, Madden C, Beshay J, White J, Gambrell K, Rickert K. Autologous and acrylic cranioplasty: A review of 10 years and 258 cases. World Neurosurg. 2014. 82: e525-30
7. Mezue WC, Ndubuisi C, Ohaegbulam SC, Chikani M, Erechukwu U. Cranial bony decompressions in the management of head injuries: Decompressive craniotomy or craniectomy?. Niger J Clin Pract. 2013. 16: 343-7
8. Nagy A, Bognar L, Pataki I, Barta Z, Novak L. Ventriculosubgaleal shunt in the treatment of posthemorrhagic and postinfectious hydrocephalus of premature infants. Childs Nerv Syst. 2013. 29: 413-8
9. Ng ZY, Ang WJ, Nawaz I. Computer-designed polyetheretherketone implants versus titanium mesh (± acrylic cement) in alloplastic cranioplasty: A retrospective single-surgeon, single-center study. J Craniofac Surg. 2014. 25: e185-9
10. Panczykowski DM, Okonkwo DO. Premorbid oral antithrombotic therapy and risk for reaccumulation, reoperation, and mortality in acute subdural hematomas. J Neurosurg. 2011. 114: 47-52
11. Schmidt JH, Reyes BJ, Fischer R, Flaherty SK. Use of hinge craniotomy for cerebral decompression. Technical note. J Neurosurg. 2007. 107: 678-82
12. Schoekler B, Trummer M. Prediction parameters of bone flap resorption following cranioplasty with autologous bone. Clin Neurol Neurosurg. 2014. 120: 64-7
13. Senft C, Schuster T, Forster MT, Seifert V, Gerlach R. Management and outcome of patients with acute traumatic subdural hematomas and pre-injury oral anticoagulation therapy. Neurol Res. 2009. 31: 1012-8
14. Sucu HK, Gokmen M, Gelal F. The value of XYZ/2 technique compared with computer-assisted volumetric analysis to estimate the volume of chronic subdural hematoma. Stroke. 2005. 36: 998-1000
15. Thien A, King NK, Ang BT, Wang E, Ng I. Comparison of polyetheretherketone and titanium cranioplasty after decompressive craniectomy. World Neurosurg. 2015. 83: 176-80
16. Valença MM, Martins C, da Silva JC. “In-window” craniotomy and “bridgelike” duraplasty: An alternative to decompressive hemicraniectomy. J Neurosurg. 2010. 113: 982-9
17. Zanaty M, Chalouhi N, Starke RM, Chitale R, Hann S, Bovenzi CD. Predictors of infections following cranioplasty: A retrospective review of a large single center study. Scientific World Journal 2014. 2014. p.