- Department of Pediatric Neurosurgery, Mexican Institute of Social Security, “La Raza” Medical Center, Mexico City, Mexico.
- Department of Fetal Surgery, Mexican Institute of Social Security, “La Raza” Medical Center, Mexico City, Mexico.
- Department of Neurosurgery, Mexican Institute of Social Security, “La Raza” Medical Center, Mexico City, Mexico.
- Department of Neurosurgery, University Hospitals, Leuven, Belgium
- MyFetUZ Fetal Research Center, KU Leuven, Belgium
Correspondence Address:
Antonio García Méndez, Department of Pediatric Neurosurgery, Mexican Institute of Social Security, “La Raza” Medical Center, Mexico City, Mecixo.
DOI:
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio García Méndez1, Antonio Helue Mena2, Fernando Agustín Aguilar1, Jorge Alberto Rivera Segura1, Miguel Ángel García Guerrero3. Fetal surgery for occipital encephalocele: A case report. 22-Dec-2023;14:433
How to cite this URL: Antonio García Méndez1, Antonio Helue Mena2, Fernando Agustín Aguilar1, Jorge Alberto Rivera Segura1, Miguel Ángel García Guerrero3. Fetal surgery for occipital encephalocele: A case report. 22-Dec-2023;14:433. Available from: https://surgicalneurologyint.com/surgicalint-articles/12688/
Abstract
Background: Occipital encephalocele is a congenital defect of the neural tube at the level of the cranial midline, which results in herniation of meninges and brain tissue. The results of the management of myelomeningocele study determine the maternal and fetal risks for an open fetal surgery and have motivated the constant review of the concepts and strategies which the pediatric neurosurgeon can employ for the treatment of neural tube defects in the prenatal period.
Case Description: We present a case of a female patient in utero of 26 gestational weeks with the diagnosis of an occipital encephalocele treated by open fetal surgery. During week 20 of gestation, the diagnosis of occipital encephalocele was made by ultrasound, which was corroborated by fetal magnetic resonance that showed cranial protrusion of neural and meningeal content in the occipital region, measuring 1.6 × 2.8 × 3.3 cm with an approximate volume of 7.7 cc through a bone defect of 6 mm. The closure of the defect was performed by the postnatal surgical technique adapted to the open fetal surgery. Later, the patient was born transabdominal with a 2.8 cm occipital wound, with suture points and approximated borders, normocephalic, without clinical signs of sepsis, hydrocephalus, or overt neurologic compromise.
Conclusion: Open fetal surgery is a therapeutic option in the face of an isolated occipital encephalocele. This case report demonstrates the viability of the surgical procedure by the adaptation of a postnatal surgical technique to a prenatal surgery. Further studies are needed to evaluate the long-term functional results, comparing them with those seen in patients who undergo a postnatal procedure.
Keywords: Fetal surgery, Cranial dysraphism, Occipital encephalocele
INTRODUCTION
Occipital encephalocele is a congenital defect of the neural tube at the level of the cranial midline, which results in herniation of the meninges and cerebral tissue.[
Encephalocele is an anomaly of the embryonic mesoderm resulting from a faulty differentiation of the superficial ectoderm with the neural ectoderm.[
Although it can occur as an isolated disorder, it can also be associated with other congenital anomalies or genetic syndromes.[
Ultrasound is sufficient for an accurate diagnosis with a sensitivity detection of over 90% which can be supplemented or not with a fetal magnetic resonance.[
The results of the management of myelomeningocele study (MOMS study) determine the maternal and fetal risks for open fetal surgery and have motivated the constant review of the concepts and strategies which the pediatric neurosurgeon can employ for the treatment of neural tube defects in the prenatal period.[
CASE DESCRIPTION
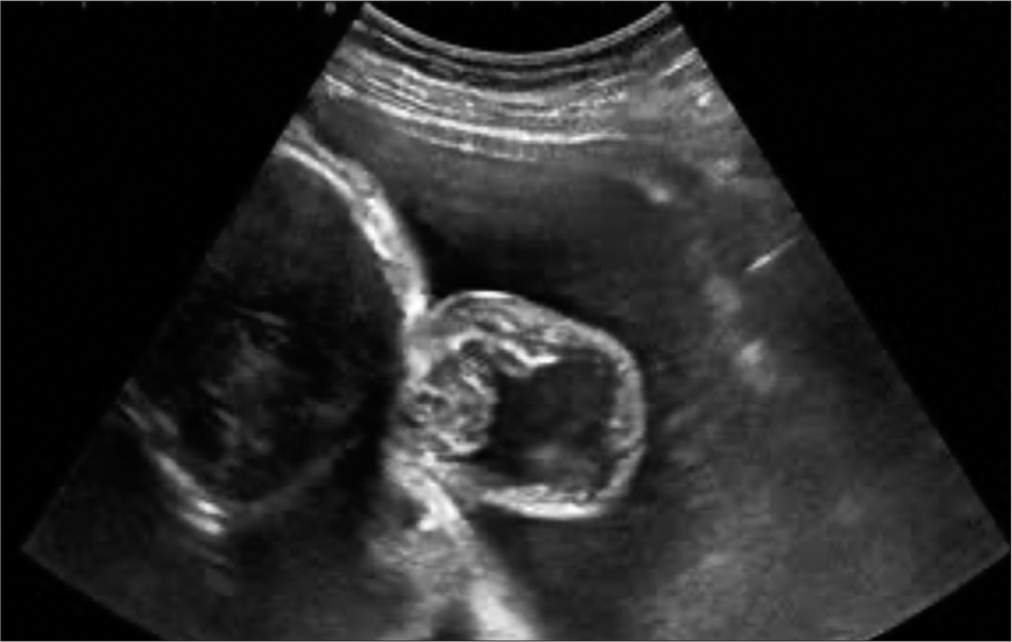
Female patient in utero of 26 weeks’ gestation by obstetric ultrasound product of a third pregnancy from a 30-year-old woman with a history of gestational diabetes diagnosed at 12 weeks’ gestation, treated with Metformin. During week 20 of gestation, an obstetric ultrasound is performed, which reveals an occipital encephalocele [
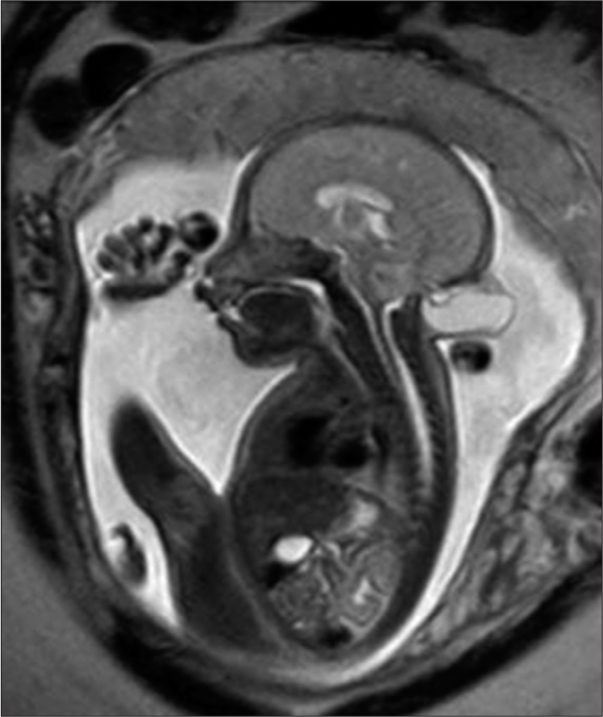
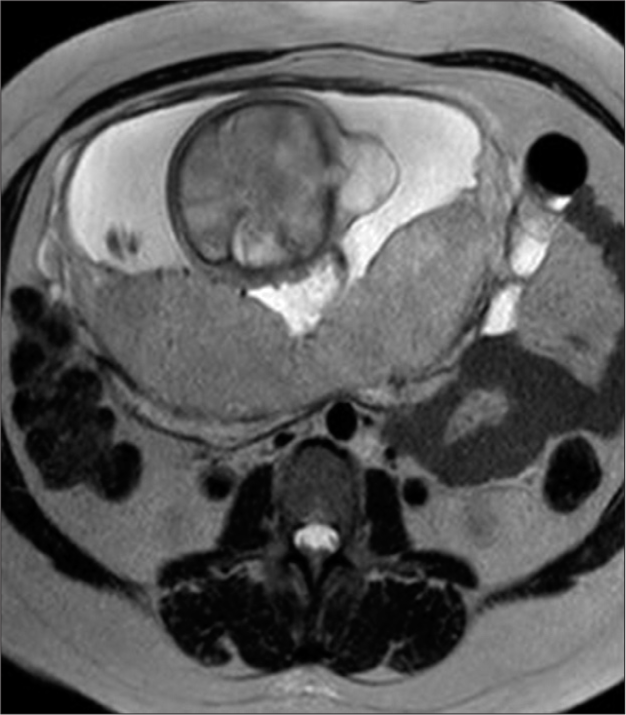
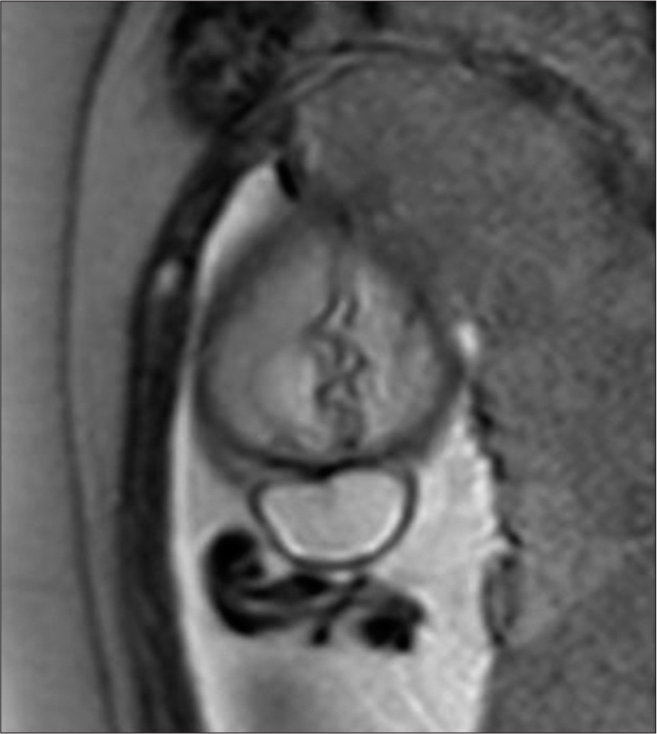
A fetal magnetic resonance scan demonstrated a single product of approximately 26 weeks by fetometry with a midline cranial protrusion of neural and meningeal content similar to cerebrospinal fluid (CSF) in occipital region measuring 1.6 × 2.8 × 3.3 cm and an estimated volume of 7.7 cc as well as an occipital bone defect of 6 mm [
The multidisciplinary team evaluated the potential risks described for open fetal surgery and, along with the ethics committee of the hospital institution and the signed informed consent, decided to perform the postnatal surgical technique for the repair of an occipital encephalocele adapted to an in-utero procedure.
Surgical technique
The surgical procedure was performed at the National Medical Center La Raza Mexican Institute for Social Security in Mexico City on April 27, 2023. The steps followed in the surgical obstetric technique were like those proposed by Adzick et al. in the MOMS study.[
The abdominal wall is opened with an extended Pfannenstiel incision; the gravid uterus is extracted from the abdominal cavity, followed by a detailed mapping of placenta localization and identification of the parts of the umbilical cord by means of a transoperative echography. The site of the hysterotomy depends on the placenta localization. A 3 cm longitudinal mini-hysterotomy was performed [
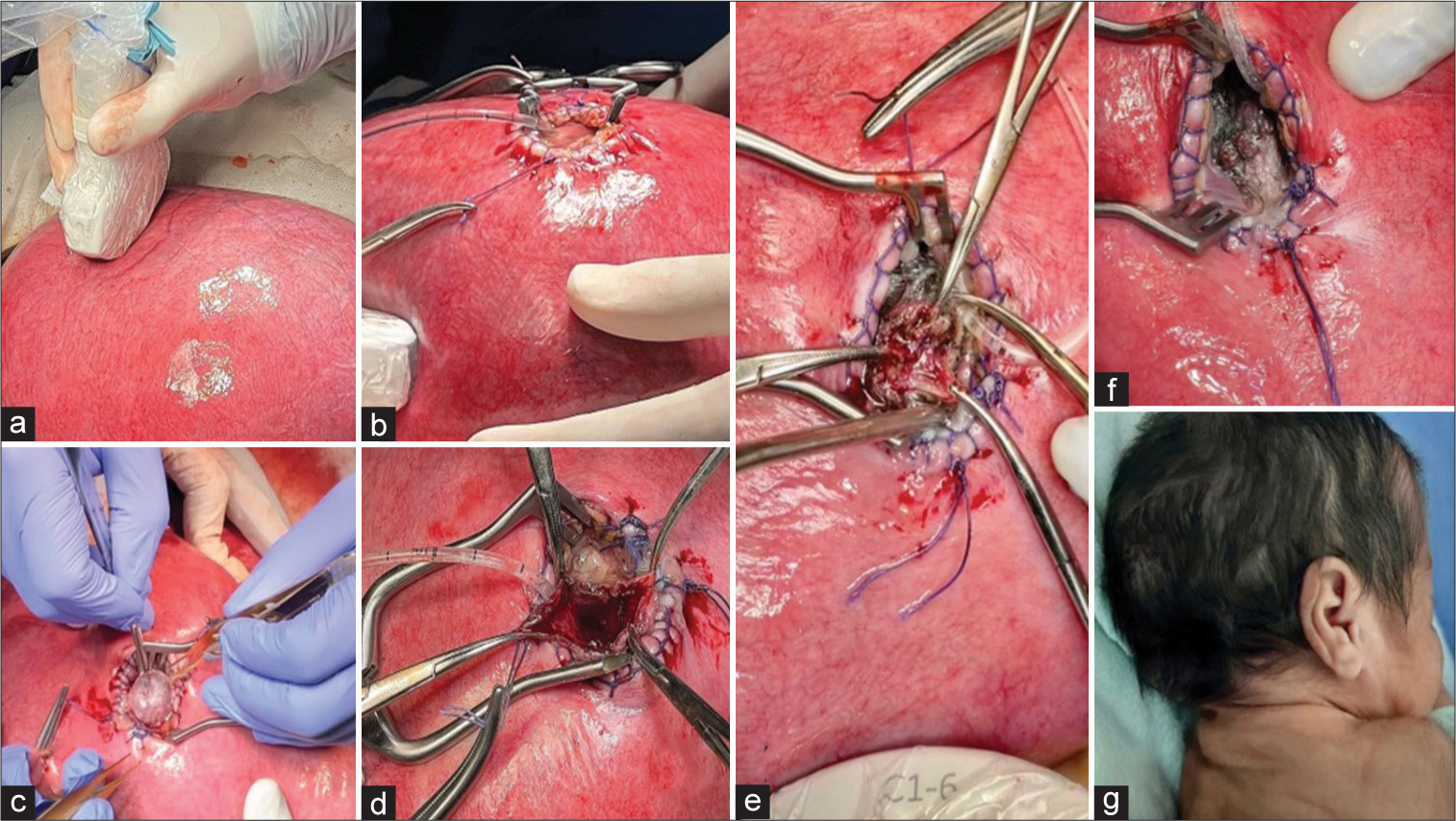
Figure 5:
Surgical technique. (a) Localization of placenta by transoperative echography, planning of mini hysterotomy. (b) A 3 cm mini hysterotomy and widening by a Weitlaner retractor. (c) Exposure of occipital encephalocele. (d) Midline incision of encephalocele sac and exposure of meninges. (e) Tight closure of meninges by a 5-0 propylene running suture. (f) Complete closure of occipital defect without leak of cerebrospinal fluid (CSF). (g) Postnatal wound of clinical case with borders closed with a running suture, without leak of CSF.
The neural surgical steps begin with the positioning of the head of the fetus on the site of the uterine opening guided by transoperative ultrasound. The repair of the encephalocele starts with a midline incision of the hernial sac [
A safety margin of 1.5 cm was maintained in the meningeal layer to have a tight closure without complications. The protruded neural tissue was returned to its intracranial position, and a tight closure of the meningeal layers with a 5-0 propylene running suture was completed [
The cutaneous remnant was excised, and the skin was reconstructed, avoiding redundant tissue with a 25 3-0 poliglecaprone running suture. After the repair of the occipital encephalocele, the fetus was returned to the uterine cavity [
FOLLOW-UP AND RESULTS
Four weeks after fetal surgery, the mother entered the operating room with the following diagnoses: Pregnancy of 30.4 weeks gestation and gestational diabetes. A premature female patient was born transabdominally, with regular respiratory effort and a cardiac frequency of over 100 beats/min. Capurro of 30 weeks gestation, Silverman-Anderson O with a weight of 1480 g, height of 36 cm, and a cephalic perimeter of 26 cm with a 2.8 cm was obtained at birth. The wound in the occipital region with suture points and borders closed with a running suture without a leak of CSF or blood [
She is hospitalized for three weeks, reaching the ideal weight for dismissal, without presenting clinical data of neonatal sepsis, hydrocephalus or overt neurologic compromise and having adequate respiratory effort.
After one month of follow-up, the patient is active, reactive to stimuli, normocephalic, with rhomboidal anterior fontanelle, sharply pointed posterior fontanelle, short neck with redundant skin, and a close tight wound in the occipital region with adequate healing.
DISCUSSION
Encephalocele comprises 10–20% of all craniospinal dysraphisms.[
Sixty per cent of patients with an occipital encephalocele are going to present another malformation and/or chromosomic defect, and at least 15–20% of newborns will have other anomalies such as defects of the neural tube, microcephaly, Chiari malformation type 2 or 3, craniosynostosis, and syringomyelia.[
Signs and symptoms may vary significantly depending on the size, localization, and type of content which protrudes through the bone defect.[
Prenatal diagnosis can be accomplished with a three-dimensional ultrasound even from week 11 of gestation, but in the majority of cases the diagnosis is made in the second trimester.[
Ultrasound is sufficient for an accurate diagnosis with a detection sensitivity greater than 90%, although it can be complemented or not with a fetal magnetic resonance.[
The latter is indicated when the processes of maturation and neuronal myelination need to be evaluated.[
The most important thing in the treatment of this pathology is the adequate prenatal diagnosis utilizing all the ancillary laboratory tests available, such as blood makers, obstetric ultrasound, magnetic resonance, and fetal karyotype.[
Surgery remains the only treatment for encephalocele.[
Surgical indication must be considered in cases where there is a risk of rupture of the sac and/or CSF fistula, meningitis, the content of herniated tissue, presence of vascular structures, associated hydrocephalus, and cosmetic appearance.[
The complications that accompany postnatal surgery of an occipital encephalocele include the presence of postsurgical meningitis, CSF fistula, hydrocephalus, wound infection, wound dehiscence, and death.[
Fetal surgery is performed in many centers around the world for a great number of pathologies.[
The surgical repair of an occipital encephalocele is not an experimental procedure because it is the same technique utilized in the postnatal period: early identification of the meningeal layers, preservation of neural tissue (whenever possible), and tight closure of the dura mater and skin.[
At present, there are no inclusion or exclusion criteria for the selection of candidates for fetal surgery of an isolated occipital encephalocele. However, there is evidence in the literature about recommendations such as gestational age between 20 and 27 weeks gestation, mother’s age equal to or over 18 years, normal fetal karyotype, and cystic hernial sac with <20% of neural tissue.[
Furthermore, there are recommendations which contraindicate the treatment in utero: the presence of syndromic fetal anomalies, chromosomopathies, neural tissue >20% of the volume of hernial sac, and the presence of vascular structures or from the encephalic trunk within the sac.[
On the other hand, other criteria that should be considered in the preoperative assessment are the following: risk for premature delivery due to adverse conditions of the mother or the course of pregnancy such as short cervix, placenta previa, oligohydramnios, chorioamnionitis, uncontrolled gestational diabetes or arterial hypertension, human immunodeficiency virus infection, hepatitis B or C, and refusal of the procedure.[
The maternal and fetal risks that are well-defined for the procedure by an open fetal surgery should be balanced and clearly focus on the benefit of the fetus.[
The only drawback of the report is that the patient was born prematurely to the expected casued by multifactoral conditions. However, she did not present complications inherent to a postnatal occipital encephalocele, completed her scheme of pulmonary maturation, and once the ideal weight was reached, she was discharged home without an overt neurologic deficit.
Fetal surgery requires the active participation of a multidisciplinary group.[
The performance of some critical steps, such as optimal localization of the hysterotomy, opening of a gravid uterus with minimal bleeding, fetal stabilization throughout the procedure, and intraoperative tocolysis before extubation, permits a successful evolution of the binomial.[
It is important to emphasize the necessity of ample experience with prenatal and postnatal neurosurgical procedures for neural tube defects.[
Cavalheiro et al. suggest the placement of an absorbable miniplate between the bone and dura, with the theoretical objective of limiting the pressure on the defect and preventing the progression of the encephalocele. One of the advantages of carrying out the closure of the defect in utero is the easy manipulation of the hernial sac, which is smaller compared to that of the postnatal period, and the amount of neural tissue is less, allowing the reintegration into the cranial cavity.[
Cavalheiro et al. compare the results of patients who underwent fetal surgery versus patients operated on during the postnatal period, reporting that fetal surgery is associated with a better prognosis in cognitive development.[
The results are encouraging, particularly if the herniated neural content is below 20% of the total volume of the encephalocele.[
Progressive herniation in utero plays an important role in the development of the clinical prognosis.[
An additional investigation is necessary, including the use of a larger database and studies in animals to demonstrate the progressive hernia of functional neural tissue, which later presents damage within the uterine cavity, as well as the role it plays in the pathophysiology of an occipital encephalocele.
CONCLUSION
Open fetal surgery is a therapeutic option in the face of an isolated occipital encephalocele. This report demonstrates the viability of the surgical procedure by the adaptation of the postnatal technique to the prenatal surgery, with the aim of expanding the alternatives for the treatment of neural tube defects.
Further studies are needed to evaluate long-term functional results and demonstrate whether the procedure in utero plays an important role in the modulation of progressive herniation of neural tissue and in the prevention of postnatal complications such as hydrocephalus or seizures hard-to-control.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
1. Adzick NS, Thom EA, Spong CY, Brock JW, Burrows PK, Johnson MP. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011. 364: 993-1004
2. Cavalheiro S, da Costa MD, Barbosa MM, Suriano IC, Ottaiano AC, de Andrade Lourenção Freddi T. Fetal neurosurgery. Childs Nerv Syst. 2023. 39: 2899-9
3. Cavalheiro S, Silva da Costa MD, Nicácio JM, Dastoli PA, Capraro Suriano I, Barbosa MM. Fetal surgery for occipital encephalocele. J Neurosurg Pediatr. 2020. 26: 605-12
4. Cavalheiro S, da Costa MD, Mendonça JN, Dastoli PA, Suriano IC, Barbosa MM. Antenatal management of fetal neurosurgical diseases. Childs Nerv Syst. 2017. 33: 1125-41
5. Gadgil N, McClugage SG, Aldave G, Bauer DF, Weiner HL, Huisman TA. Natural history of posterior fetal cephaloceles and incidence of progressive cephalocele herniation. J Neurosurg Pediatr. 2022. 30: 1-7
6. Hogan A, Ullmer N. Fetal occipital encephalocele: A case report. J Diagn Med Sonography. 2022. 38: 264-9
7. Markovic I, Bosnjakovic P, Milenkovic Z. Occipital encephalocele: Cause, incidence, neuroimaging and surgical management. Curr Pediatr Rev. 2020. 16: 200-5
8. Meller C, Covini D, Aiello H, Izbizky G, Portillo Medina S, Otaño L. Update on prenatal diagnosis and fetal surgery for myelomeningocele. Actualización del diagnóstico prenatal y cirugía fetal del mielomeningocele. Arch Argent Pediatr. 2021. 119: e215-28
9. Milani HJ, Barbosa MM, Sarmiento S, Cavalheiro S. Intrauterine open fetal surgery for correction of isolated occipital encephalocele. Ultrasound Obstet Gynecol. 2017. 50: 1-47
10. Moron AF, Barbosa MM, Milani HJ, Sarmento S, Kusano CU, Munhoz P. OC16.05. Open fetal surgery for congenital malformations: Feasibility and perinatal outcomes. Ultrasound Obstet Gynecol. 2017. 50: 34-4
11. Protzenko T, Dos Santos Gomes Junior SC, Bellas A, Salomão JF. Hydrocephalus and occipital encephaloceles: Presentation of a series and review of the literature. Childs Nerv Syst. 2021. 37: 3437-45
The authors describe a case of occipital encephalocele that was operated in utero. Using their personal experience in fetal myelomeningocele (MMC) repair and the postnatal surgery of encephalocele, and following the pioneering work of Sergio Cavalheiro[
However, some comments have to be made to put this case into proper perspective. Fetal surgery for MMC has a solid pathophysiological basis: direct exposure of the placode to the neurotoxic amniotic fluid leads to progressive damage to the neurons throughout the pregnancy, and persistent leakage of cerebrospinal fluid (CSF) leads to tonsillar herniation, which plays a role in the hydrocephalus seen in almost all children with MMC. Fetal surgery aims to reverse these factors. The fact that these objectives could be reached was shown in animal experiments before the use of the technique in humans. The randomized MOMS trial[
Occipital encephalocele is a different disorder: it is a closed defect without CSF leakage and exposure of the brain tissue to the amniotic fluid. The natural history during the fetal period is not well known, and although some cases are clearly progressive, others definitely are not. There is no clear definition of progression: a larger fluid volume without damage to the brain tissue, e.g., is not the same as progressive herniation of brain tissue. There are no good surgical animal models of encephalocele[
One other aspect also should be considered: ‘fetal surgery’ is not only fetal surgery but also maternal and uterine surgery. The risks to the mother are not negligible (chorioamniotic membrane separation, spontaneous rupture of membranes, uterine rupture, infection, and even maternal death), and future pregnancies are compromised. From an ethical point of view, it is very difficult to determine beneficence and non-maleficence when one has to consider both the mother and the fetus. As a minimum and in every case, not only the consent of the pregnant woman, who is in a vulnerable position and under a lot of emotional stress to make a decision but also the approval of a well-informed ethical review board should be sought before considering this surgery.
In summary, occipital encephalocele is a disorder for which more (pre)clinical studies are highly needed—the present case by Dr. García Méndez and his colleagues shows that fetal surgery for occipital encephalocele is feasible but does not offer scientific proof that this is the procedure of choice.
References
1. Adzick NS, Thom EA, Spong CY, Brock JW, Burrows PK, Johnson MP. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011. 364: 993-1004
2. Cavalheiro S, Silva de Costa MD, Nicácio JM, Dastoli PA, Suriano IC, Barbosa MM. Fetal surgery for occipital encephalocele. J Neurosurg Pediatr. 2020. 26: 605-12
3. Rolo A, Galea GL, Savery D, Greene ND, Copp AJ. Novel mouse model of encephalocele: Post-neurulation origin and relationship to open neural tube defects. Dis Model Mech. 2019. 12: dmm040683






Rodrigo Ramos-Zúñiga
Posted January 18, 2024, 12:06 pm
It is a systematic multidisciplinary process that fortunately is now part of the alternatives in fetal surgery for the benefit of the patient. The risk-benefit balance including maternal health is primordial. The approval of the ethics committee is necessary in these cases as a clarification of what is indicated in the final statements of the article, as these are people ( Both) in a vulnerable condition.