- Hand and Microsurgical Center, 2nd Affiliated Hospital, Harbin Medical University, Harbin, China
- State-Province Key Laboratories of Biomedicine-Pharmaceutics, Harbin Medical University, Harbin, China
- Heilongjiang Medical Science Institute, Harbin Medical University, Harbin, China
- Department of Molecular Pharmacology and Therapeutics, Stritch School of Medicine, Loyola University, Chicago, Illinois, USA
- Department of Anatomy, Harbin Medical University, Harbin, China
- Department of Radiology, 2nd Affiliated Hospital, Harbin Medical University, Harbin, China
- Turin Advanced Neuromodulation Group, Turin, Italy, Harbin Medical University, Harbin, China
Correspondence Address:
Xiaoping Ren
Turin Advanced Neuromodulation Group, Turin, Italy, Harbin Medical University, Harbin, China
DOI:10.4103/sni.sni_415_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Xiaoping Ren, Ming Li, Xin Zhao, Zehan Liu, Shuai Ren, Yafang Zhang, Shide Zhang, Sergio Canavero. First cephalosomatic anastomosis in a human model. 17-Nov-2017;8:276
How to cite this URL: Xiaoping Ren, Ming Li, Xin Zhao, Zehan Liu, Shuai Ren, Yafang Zhang, Shide Zhang, Sergio Canavero. First cephalosomatic anastomosis in a human model. 17-Nov-2017;8:276. Available from: http://surgicalneurologyint.com/surgicalint-articles/first-cephalosomatic-anastomosis-in-a-human-model/
Abstract
Background:Cephalosomatic anastomosis (CSA) has never been attempted before in man as the transected spinal cords of the body donor and body recipient could not be “fused” back together. Recent advances made this possible. Here, we report on the surgical steps necessary to reconnect a head to a body at the cervical level.
Methods:Full rehearsal of a CSA on two recently deceased human cadavers was performed at Harbin Medical University, Harbin, China.
Results:The surgery took 18 hours to complete within the time frame planned for this surgery. Several advances resulted from this rehearsal, including optimization of the surgical steps, sparing of the main nerves (phrenics, recurrent laryngeal nerves), and assessment of vertebral stabilization.
Conclusion:Several specialties are involved in a full-scale CSA, including neck surgery, vascular surgery, orthopedic surgery, plastic surgery, gastrointestinal surgery, and neurosurgery, as well as the operating staff. This rehearsal confirmed the surgical feasibility of a human CSA and further validated the surgical plan. Education and coordination of all the operating teams and coordination of the operative staff was achieved in preparation for the live human CSA.
Keywords: Cephalosomatic anastomosis, GEMINI, head transplant, spinal cord fusion, spinal fixation, vascular reconnection
INTRODUCTION
A human cephalosomatic anastomosis (CSA) (also referred to as a head transplantation or a body transplantation depending on one's own point of view) has been suggested as the only therapeutic option for a select group of peripheral neuromuscular diseases which up to now are incurable by any other means.[
In 1970, the first experimental model of a CSA in a primate was reported; however, only the blood supply to the brain from the donor body was re-established; the spinal cord and the continuity of other organs were not restored. This experiment was designed to confirm that deep hypothermia can protect the brain and preserve brain function after re-warming during cephalic exchange. Indeed, recovery/preservation of certain behavioral characteristics and functions controlled purely by the recipient head was also demonstrated.[
In this paper, we will refer to the healthy head receiving a healthy body as the Recipient (R) and the body without its head that will be attached to R as the Donor (D). D is a brain-dead organ donor whose family consented to organ or – in this case – body donation.
A CSA is a multi-specialty procedure that requires interdisciplinary expertise. Several aspects are of key importance from a logistic standpoint:
Organization of the operating team and executing the planned operative procedure: it is imperative to develop an entire team (s) and assess the number/specialty of surgeons necessary to complete the procedure and to learn how to “interweave” and coordinate their parts of the procedure seamlessly, thereby minimizing the duration of the CSA; this orchestration of surgical teams needs to be carried out simultaneously, with one surgical team working on the R and another on the body donor D. Perfection of details of the operative procedure specifically related to human. Additional steps were necessary to rehearse in the human cadaver. It was necessary to coordinate procedures on the anterior and posterior compartments of the neck in the cadavers. Other points concern the selection of the best planes of separation of R's head from the diseased R's body and the evaluation of the best spinal fixation system. Equally important is the preservation of selected nerves from R and D to allow vocal cord function and spontaneous breathing, as well as the reconstruction of the other organ systems disrupted by the CSA. More specifically, the goal is to transfer the Recipient's head along with R's larynx, including the recurrent laryngeal nerves: these nerves will be left intact in toto to assure that phonation is preserved. This step is crucial to be able to communicate with R after the surgery.
Our starting hypothesis was that a successful CSA can be only achieved by having the surgeons acquire hands-on expertise on an actual CSA performed on cadavers. Unlike other transplantation procedures involving a single organ (heart, liver, kidney, lung, pancreas), CSA requires a unique coordination of neurologic, vascular, orthopedic, gastrointestinal, and cardiothoracic surgeons working together.
This paper summarizes the operative steps involved in a human CSA based on a full-scale “rehearsal” of the actual procedure on cadavers. Several selected aspects of the procedure will be discussed.
We will not comment on the ethics of the procedure, as this is exhaustively treated elsewhere.[
MATERIALS AND METHODS
This study was approved by the Human Research Ethics Board, Harbin Medical University, PRC, and the patients (who donated their bodies for research; families signed a consent for such experimental work).
A preliminary series of experiments on fresh human cadavers was conducted to assess the appropriate surgical planes of dissection for a full CSA (unpublished observations). Only at this point was the decision made to proceed with a full rehearsal. Two fresh male cadavers of similar build were provided by the Institute of Anatomy, HMU, Harbin, PRC with appropriate IRB approval and patients’ families consent.
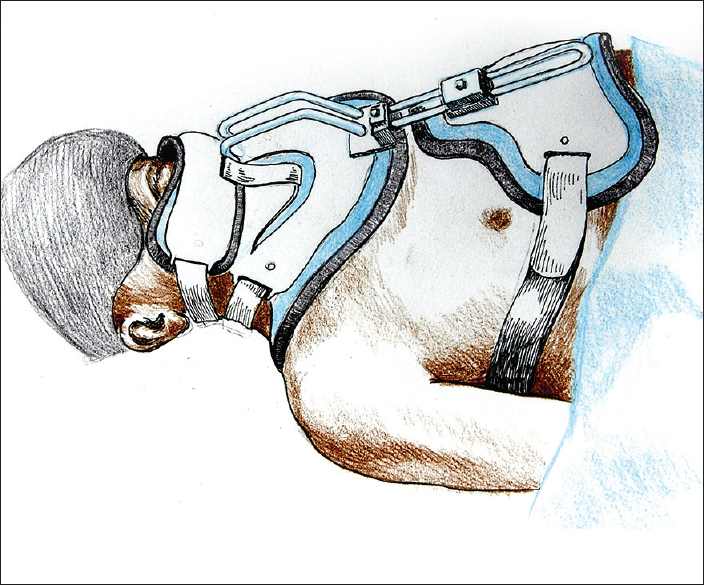
Although CSA on living subjects will be conducted in a standard neurosurgical sitting position, in this trial rehearsal, the two cadavers were aligned on two adjoining tables and kept supine for the anterior approach and then rolled over for the posterior approach. Two teams of 5 surgeons worked simultaneously on the two cadavers to prepare D's body and R's head; no low tracheostomies were fashioned as would be necessary in the actual scenario.[
PROCEDURE
Anterior approach (D)
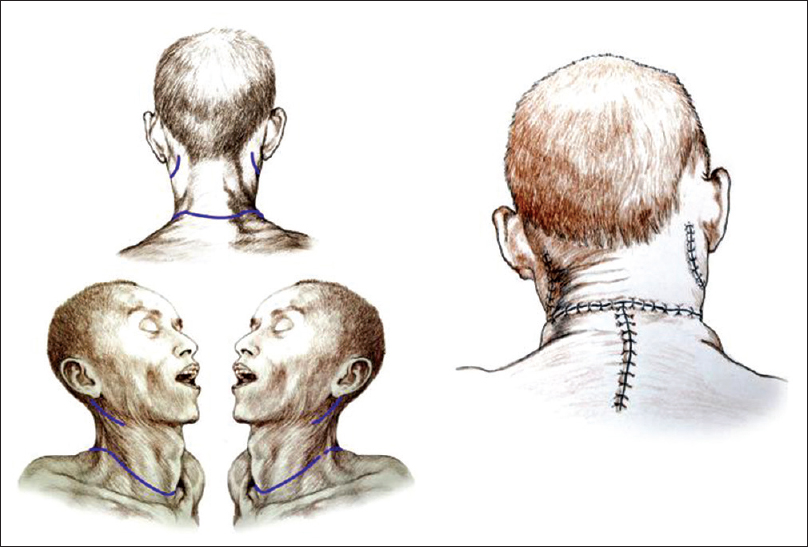
Preparation of D's body was facilitated by extending the neck with two pads underneath the shoulders. A transverse cervical incision was fashioned 3 cm caudal to the cricoid cartilage, followed by separation of the subcutaneous tissues and the platysma, similar to a standard Kocher incision [
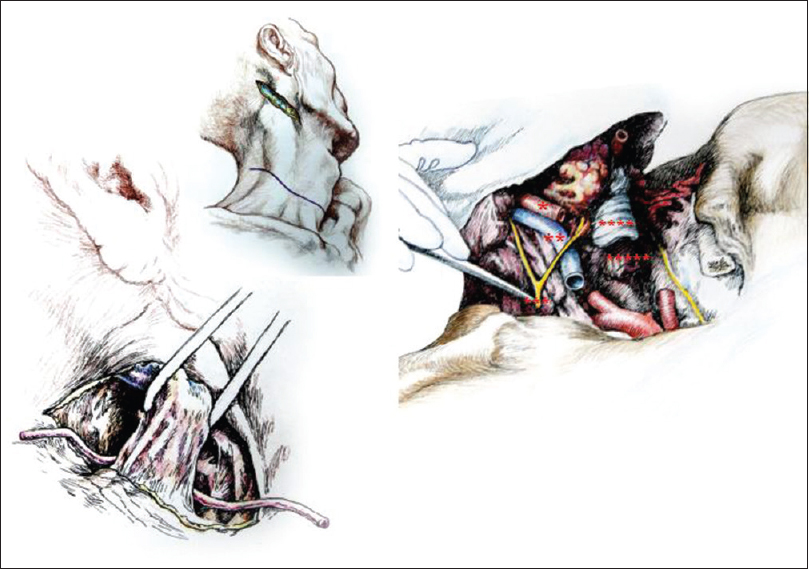
At this point, access to the planes of the underlying vascular and nervous structures was unobstructed. Careful dissection exposed the carotid sheaths 2 cm cranial to the sternum and then the common carotid artery, the internal jugular vein, the vagus nerve bilaterally. The vagi and the recurrent laryngeal nerves (RLN) were dissected free and transected [the distal extension (loop) of the RLN were left in place]. The phrenic nerves coursing on the anterior scalene muscle were purposely spared, but the component arising from the C3 root was transected close to the point of exit from the intervertebral foramen while carefully sparing injury to the C4 and C5 roots (see Discussion). Next, the thyroid gland, trachea, and esophagus were isolated. In particular, the thyroid was kept in place but peeled from the underlying plane after ligating and transecting the superior thyroid arteries and veins. The trachea and then the esophagus were transected horizontally at the level of C5-6. The bellies of the anterior and middle scalene muscles were transected cranial to their points of insertion on the first rib and lifted up. The transverse cervical arteries were visualized and left intact.
Full access to the anterior aspect of the cervical vertebral spine was obtained [
Anterior approach (R, which includes the head to be transplanted)
The approach was similar, however, several notable differences must be mentioned. The ansae cervicalis (hypoglossi) were exposed superficial to the internal jugular veins and spared. The greater auricular nerve was spared by transecting the sternocleidomastoids immediately caudal to the nerve. The accessory nerves were visualized and spared. The thyroid was removed completely. The need to spare the RLNs in toto (to allow the patient to vocalize on reawakening) required a downward extension of the dissection into the mediastinum. Thus, the ends of the clavicles close to their junction with the manubrium were exposed and wire-sawed off for approximately 2 cm, along with part of the sternal manubrium after the infrahyoid muscles were detached from their claviculosternal insertions. The RLNs were carefully identified and dissected free along their course. After clamping and transecting the right subclavian artery proximally (to preserve the right RLN), and immediately thereafter the aortic arch (to preserve the left RLN), around which the RLNs loop, the RLN were split from the rest of the vagus and kept intact. At this point, both RLNs were mobilized to lie close to the larynx. A saline-soaked gauze protected the nerves during further operative maneuvers. Particular attention at this point went to preserve Pirogoff's angles, i.e. the junction of the internal jugular and subclavian veins at both sides of the neck, where lymph from the thoracic duct – on the left – and lymphatic trunk – on the right – enters the venous circulation.
Posterior approach
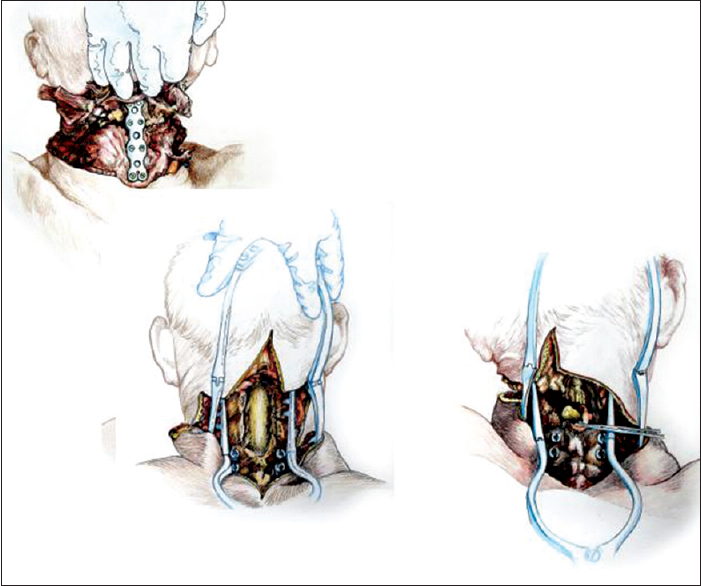
After rolling over both R and D in a prone position, a longitudinal midline incision was made extending down to the first dorsal spinous processes and then the incision was connected transversly to the anterior skin incision. The upper part of the trapezius was transected and detached from its insertion bilaterally, followed by similar treatment of the semispinalis capitis and cervicis, splenius capitis and cervicis, longissimus capitis, and the levator scapulae. In particular, the paraspinal muscles were stripped from the spinous processes as well as from the facet joints of C2-6. A standard C2-5 laminectomy followed. At this point, after drilling into the lateral masses of C3-4 in R and C4-5 in D, screws were installed in the bone. After opening the dura longitudinally and transversally, the posterior vessels were coagulated. The cord was subsequently sectioned sharply with a diamond blade (Shanghai Jingming Fine Technology Co., Shanghai, PRC) at C4 in R and C2 in D (see Discussion), with further spot coagulation of the anterior spinal vessels [
Chimeral anastomosis
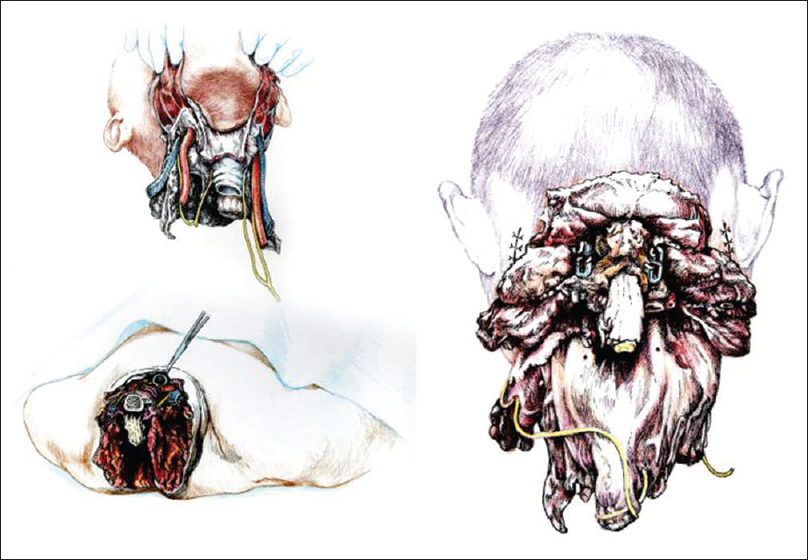
Once detached from the body, R's head was transferred on the decapitated supine D's body by aligning the transected verterbal bodies of C3 [
The chimera was then rolled over and the transected spinal cords were length-adjusted, i.e. removing the excess length of spinal cords intentionally left at the time of transection of the cords in both R and D (see Discussion for explanation) in the vertebral canal. The two stumps were sutured together, but in the real case scenario a resorbable negative pressure connector (explained in 5) will be positioned and alignment confirmed under somatosensory evoked potential guidance (SSEPs). Thereafter, the dura was closed in a standard neurosurgical water-tight fashion with nonabsorbable suture: this is a critical step to avoid a CSF leak, with its attendant risks of delayed wound healing and increase in infection rate and possible mortality. Also, CSF spaces would be refilled with saline in the real scenario. A mock plate of a spinal cord stimulator was then secured to the dura by 4 sutures: in vivo, the plate would remain in place indefinitely, while the connecting wire and the battery would be removed at the end of the rehabilitation period. (Note that in the actual scenario, spinal cord fusion [GEMINI: 3,5 will be carried out as soon as the anterior plate has been secured; all other maneuvers will follow this very important step). Both vertebral arteries were re-anastomosed (see Discussion). A CerviFix/Axon lateral mass screw-and-rod System (SYNTHES SPINE, West Chester, PA, USA) was used for posterior cervical stabilization (see Discussion). Thereafter, the posterior neck muscles were sutured and then standard layered closure (subcutaneous tissue and skin) followed [
This rehearsal operation took 18 hours to complete from first skin incision in both donor and recipient to final skin closure in the chimera.
DISCUSSION
This paper describes the first, full, cadaveric rehearsal of a human CSA in preparation for the actual in vivo operation. All operative maneuvers were performed without hindrance from pathologic lesions or embryologic anomalies in the subjects’ necks, thus making the process quite smooth. Actual bleeding was not an issue in this cadaveric CSA but may add to the operating time. Nonetheless, several additional points require clarification.
Peripheral nerve sparing
The priority during a CSA is sparing of the integrity, continuity, and function of the key peripheral nervous structures of both D and R as much as possible to allow prompt behavioral recovery and to spare the surgeons from time-consuming microsurgical reconnection of peripheral nerves, thereby shortening the overall surgical time. In particular, the following structures are of special interest: phrenic nerves, RLNs, vagi, and cervical plexus. The brachial plexus, which is formed in the posterior triangle of the neck by the union of C5-T1, is left undisturbed in D.
The cervical plexus is formed by the anterior rami of the C1-4 nerves. The rami are joined by connecting branches which form loops that lie in front of the origins of the levator scapulae and the scalenus medius. The plexus is covered in front by the prevertebral layer of the deep cervical fascia and is in close proximity to the internal jugular vein within the carotid sheath. A branch from C1 joins the hypoglossal nerve. Some of these C1 fibers later leave the hypoglossal nerve as the descending branch which unites with the descending cervical nerves (C2-3) to form the ansa cervicalis to supply the omohyoid, sternohyoid, and sternothyroid muscles. Because of these anatomic considerations, one would conclude that transection of the spinal cord would be best carried out at the watershed between the C4 root and C5 root, thereby leaving the cervical and brachial plexus unscathed, respectively, in R and D. However, this consideration would preclude the even more vital question of how to preserve the function of the phrenic nerves.
The phrenic nerves arise from the anterior rami of C3-5 nerves and are the sole nerve supply to the diaphragm, each nerve supplying the ipsilateral hemidiaphragm. About one-third of persons have an accessory phrenic nerve. The C5 root may be incorporated in the nerve to the subclavius muscle and may join the main phrenic nerve trunk in the thorax. Two options were considered: transecting the phrenic nerve and then reanastomosing/fusing it during the final stages of the cephalic anastomosis or simply sparing it. These possibilities would also mean transecting the spinal cord at different levels: between C2 and C3 myelomeres or C5 and C6 myelomeres. Based on our success in reestablishing electrical continuity of the spinal cord early on within 4 weeks with behavioral recovery,[
Another very important consideration has to do with phonation. Being able to communicate immediately with the treating physicians is imperative once reawakened. The key nerves for voice generation are the RLNs. Three scenarios have been discussed by our group: (1) transecting of the donor's RLN after preserving the vagus and resuturing/fusing the stump with the correspondent transected distal nerve in the recipient; (2) using a nerve graft between the stumps; and (3) complete sparing of the RLNs. It was clear that option 3 was preferable. In this cadaveric rehearsal, the RLN was fully conserved bilaterally, lifted up, and placed close to the larynx. In other words, we are transferring the entire R's larynx and its innervation onto the donor. In the actual in vivo scenario, the left RLN loops around the aortic arch. Because the only way to dissect the nerve fully is to clamp and transect the aortic arch, which would interrupt the blood flow to the rest of the body and interfere with the intravascular catheters used to induce brain hypothermia, the step needs to be left for last, right before cephalic separation. In the unlikely eventuality that this last step proves impractical for some reason, then options 1 and 2 would be pursued.[
In the end, the only nerves that need re-anastomosis/fusion are the vagi. The time required by the vagus to recover after standard microsuturing is many months, which, given its broad spectrum of action, might compromise recovery of the chimera. We notice, however, that the transplanted heart has no post-transplantation parasympathetic innervation, similar to intestinal/multivisceral transplantation. Lack of innervation from the vagi does not affect functional survival in these kinds of transplants. In particular, relatively normal intestinal motility of the gut is restored within a month after isogenic intestinal transplantation due to the adaptation and plasticity of the enteric nervous system; interestingly, late extrinsic reinnervation plays a negligible role.[
Head stability
Stability of the head and spine is a major concern to ensure successful spinal cord fusion. In this study, a combined anterior and posterior internal fixation afforded excellent stability, as assessed by subjecting the chimera to torsion and longitudinal pulling maneuvers at the end of the rehearsal operation. The fixation system we employed (a system of lateral mass screws and rods) along with anterior plate stabilization proved easy to adapt and install. This system allows for placement of the screws into the desired entry point, after which a clamp is placed on the screw; a malleable rod is contoured and threaded through the rod connectors on the clamp, thus completing the construct.[
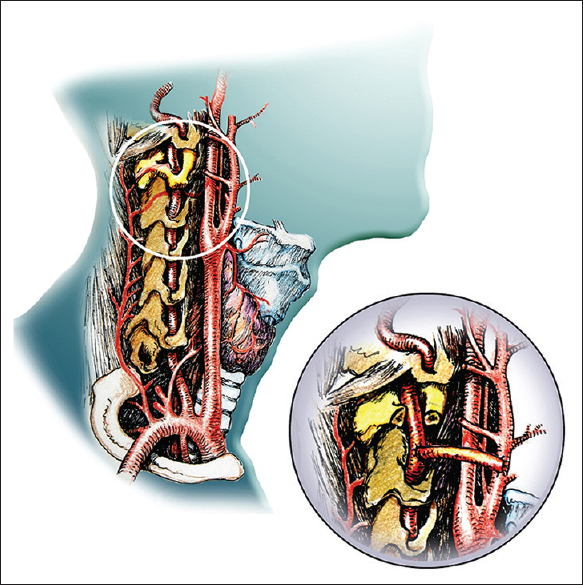
Vertebral external carotid artery bypass [ Figure 6 ]
In this first rehearsal, the vertebral arteries were reanastomosed as part of the CSA, but these anastomoses proved time-consuming. In the actual CSA, we will opt for a vertebral external carotid artery (VECA) bypass rehearsed several times in several of our previous cadaveric studies (unpublished observations), wherein a bypass between one vertebral artery and the ipsilateral external carotid artery is fashioned exploiting the previously harvested recipient's radial arteries.[
We notice in passing that our animal models clearly show that CSA can be accomplished by applying only moderate (29°C) hypothermia to R and keeping blood flowing continuously to it via cross-circulation with D.[
Spinal cord fusion (GEMINI)
As explained in previous publications,[
Logistics
In this rehearsal, the patients were lying either supine or prone, and rolled over a few times, unlike what will be necessary in the actual CSA operation when both B and D will be positioned in a standard sitting position. The sitting position is expected to facilitate simultaneous surgical maneuverability on both the posterior and anterior neck compartments; however, we are convinced that surgical maneuverability would be aided by starting with the posterior neck compartment, i.e. performing the required laminectomies/laminotomies and exposing the dura mater surrounding the cord. At this point, the neck would be slightly hyperextended, the chair reclined, and the anterior compartment approached. This sequence prevents the surgeons working on the two sides from interfering with each other.
The full-scale CSA will be conducted in a large, specially equipped operating room. The number of core personnel involved is consistent (surgeons, anesthesiologists, nurses, technicians). The need for rotating the surgeons during the operation for rest must also be an important consideration. On the basis of this rehearsal and extrapolating to the actual in vivo scenario, the entire CSA operation, in which hemostasis would add to the surgical time, may be conducted in less than 36 hours, including anesthesia and induction of hypothermia. Four separate teams of surgeons, each including 4 surgeons, is the minimum number we believe to be necessary to complete the operation safely. The specialists involved include spinal surgeons (orthopaedic or neurosurgical) and vascular, cardiothoracic, reconstructive surgeons, and general surgeons with experience/expertise in neck surgery. In the future, surgeons may be able to be trained specifically for all the aspects of the CSA.
CONCLUSION
In conclusion, this first cadaveric rehearsal confirmed that a CSA is feasible and without compelling impediments to abandon the operation. The next stage would be a full rehearsal on heart-beating brain-dead organ donors to replicate the actual operation in a live patient with a medical condition appropriate for a CSA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (81470425), the Wu Liande Foundation of HMU (Wld-qn1414), and Harbin Science and Technology Bureau (2014RFXYJ023). The authors declare no competing financial interests.
References
1. Bamba R, Waitayawinyu T, Nookala R, Riley DC, Boyer RB, Sexton KW. A novel therapy to promote axonal fusion in human digital nerves. J Trauma Acute Care Surg. 2016. 81: S177-S183
2. Camp PE. Carotid to distal vertebral artery bypass for vertebrobasilar ischemia. Case report. J Neurosurg. 1984. 60: 187-9
3. Canavero S. HEAVEN: The head anastomosis venture. Project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int. 2013. 4: S335-42
4. Canavero S, Ren X. Houston, GEMINI has landed: Spinal cord fusion achieved. Surg Neurol Int. 2016. 7: S626-8
5. Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
6. Crumley RL. Unilateral recurrent laryngeal nerve paralysis. J Voice. 1994. 8: 79-83
7. Gerke KF, Gebarski SS, Chandler WF, Phillips TW. External carotid-vertebral artery anastomosis for vertebrobasilar insufficiency. AJNR Am J Neuroradiol. 1985. 6: 33-7
8. Kim CY, Oh H, Ren X, Canavero S. Immunohistochemical evidence of axonal regrowth across polyethylene glycol-fused cervical cords in mice. Neural Regen Res. 2017. 12: 149-50
9. Li PW, Zhao X, Zhao YL, Wang BJ, Song Y, Shen ZL. A cross-circulated bicephalic model of head transplantation. CNS Neurosci Ther. 2017. 23: 535-41
10. Liu Z, Ren S, Fu K, Wu Q, Wu J, Hou L. Restoration of motor function after operative reconstruction of the acutely transected spinal cord in the canine model. Surgery. 2017. p.
11. Nandakumar R, Jagdish C, Prathibha CB, Shilpa C, Sreenivas V, Balasubramanya AM. Tracheal resection with end-to-end anastomosis for post-intubation cervical tracheal stenosis: Study of 14 cases. J Laryngol Otol. 2011. 125: 958-61
12. Oh CS, Chung IH, Koh KS, Kim HJ, Kim SS. Intradural Anastomoses Between the Accessory Nerve and the Posterior Roots of Cervical Nerves: Their Clinical Significance. Clin Anat. 2001. 14: 424-7
13. Ren XP, Song Y, Ye YJ, Li PW, Han KC, Shen ZL. Allogeneic head and body reconstruction: Mouse model. CNS Neurosci Ther. 2014. 20: 1056-60
14. Ren XP, Ye YJ, Li PW, Shen ZL, Han KC, Song Y. Head transplantation in mouse model. CNS Neurosci Ther. 2015. 21: 615-8
15. Ren X, Orlova EV, Maevsky EI, Bonicalzi V, Canavero S. Brain protection during cephalosomatic anastomosis. Surgery. 2016. 160: 5-10
16. Ren S, Liu Z, Wu Q, Fu K, Wu J, Hou LT. Polyethylene glycol-induced motor recovery after total spinal transection in rats. CNS Neurosci Ther. 2017. 23: 680-5
17. Ren XP, Canavero S. From hysteria to hope. The rise of head transplantation. Int J Surg. 2017. 41: 203-4
18. Ren XP, Canavero S. HEAVEN in the making. Between the rock (the Academe) and a hard case (a Head Transplant). Am J Bioethics Neurosci. 2017. p.
19. Shen F, Samartzis D, Fessler R.editorsTextbook of the Cervical Spine. Saunders/Elsevier; 2015. p.
20. Von Websky MW, Kalff JC, Schaefer N. Current knowledge on regulation and impairment of motility after intestinal transplantation. Curr Opin Organ Transplant. 2015. 20: 303-7
21. White RJ. Head transplants. Sci Am. 1999. p. 24-6
22. White RJ, Wolin LR, Massopust LC, Taslitz N, Verdura J. Primate cephalic transplantation: Neurogenic separation, vascular association. Transplant Proc. 1971. 3: 6024-
23. White RJ. Cerebral hypothermia and circulatory arrest. Review and commentary. Mayo Clin Proc. 1978. 53: 4508-
24. Wynn R, Har-El G, Lim JW. Tracheal resection with end-to-end anastomosis for benign tracheal stenosis. Ann Otol Rhinol Laryngol. 2004. 113: 613-7
25. Ye Y, Kim CY, Miao Q, Ren X. Fusogen-assisted rapid reconstitution of anatomophysiologic continuity of the transected spinal cord. Surgery. 2016. 160: 20-5






Flo
Posted November 22, 2017, 1:10 am
Super votre opération sera un succès, je crois en vous
Klaus H.
Posted February 4, 2018, 2:56 pm
I Think that this OP -is the right way to the Futures.