- Department of Neurosurgery, Kochi University Hospital, Nankoku, Japan.
- Department of Neurosurgery, Kochi Health Sciences Center, Kochi, Japan.
- Department of Neurosurgery, Kobe City Medical Center General Hospital, Hyogo, Japan.
Correspondence Address:
Hitoshi Fukuda, Department of Neurosurgery, Kochi University Hospital, Nankoku, Japan.
DOI:10.25259/SNI_437_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daichi Yamasaki1, Hitoshi Fukuda1, Fumihiro Hamada1, Namito Kida1, Naoki Fukui1, Kenji Okada1, Noritaka Masahira2, Tsuyoshi Ohta2, Hirotoshi Imamura3, Nobuyuki Sakai3, Tetsuya Ueba1. Flow alteration therapy for impending rupture of intracranial giant aneurysm after flow diverter placement. 29-Jul-2022;13:323
How to cite this URL: Daichi Yamasaki1, Hitoshi Fukuda1, Fumihiro Hamada1, Namito Kida1, Naoki Fukui1, Kenji Okada1, Noritaka Masahira2, Tsuyoshi Ohta2, Hirotoshi Imamura3, Nobuyuki Sakai3, Tetsuya Ueba1. Flow alteration therapy for impending rupture of intracranial giant aneurysm after flow diverter placement. 29-Jul-2022;13:323. Available from: https://surgicalneurologyint.com/surgicalint-articles/11820/
Abstract
Background: Flow diverter (FD) placement is generally effective for intractable internal carotid artery (ICA) aneurysms. However, salvage treatment for the aneurysm enlarging even after FD placement remains to be elucidated. Additional overlapping FD placement is considered the first-line treatment for residual or recurrent aneurysms. However, it is unclear whether overlapping FD is also effective for enlarging giant aneurysms that are considered impending rupture status. Although parent artery occlusion is a promising option, treatment strategy must be optimized, especially when a critical perforating artery is involved.
Case Description: A 74-year-old woman experienced rapid symptomatic growth of her giant supraclinoid ICA aneurysm 10 months after FD placement. We assumed that reinforcement of flow diverting effect alone would be less effective for this extremely intractable aneurysm with more aggressive clinical feature so that surgical bailout by parent artery occlusion was planned. Complete ICA obliteration underneath the aneurysm was unavailable due to the presence of anterior choroidal artery. Thus, we took a flow alteration strategy, where we created minimal retrograde flow through the parent artery by a combination of an extracranial-intracranial bypass and targeted endovascular proximal parent artery obliteration, resulting in prevention of aneurysmal rupture and further growth.
Conclusion: Impending rupture of the intracranial giant aneurysm after FD placement may be controllable with a tailor-made parent artery occlusion strategy even when a critical perforating artery is involved.
Keywords: Extracranial-intracranial bypass, Flow alteration therapy, Flow diverter, Giant intracranial aneurysm, Parent artery occlusion
INTRODUCTION
Recent studies have shown favorable clinical results of flow diverter (FD) placement to treat intractable intracranial aneurysms.[
CASE PRESENTATION
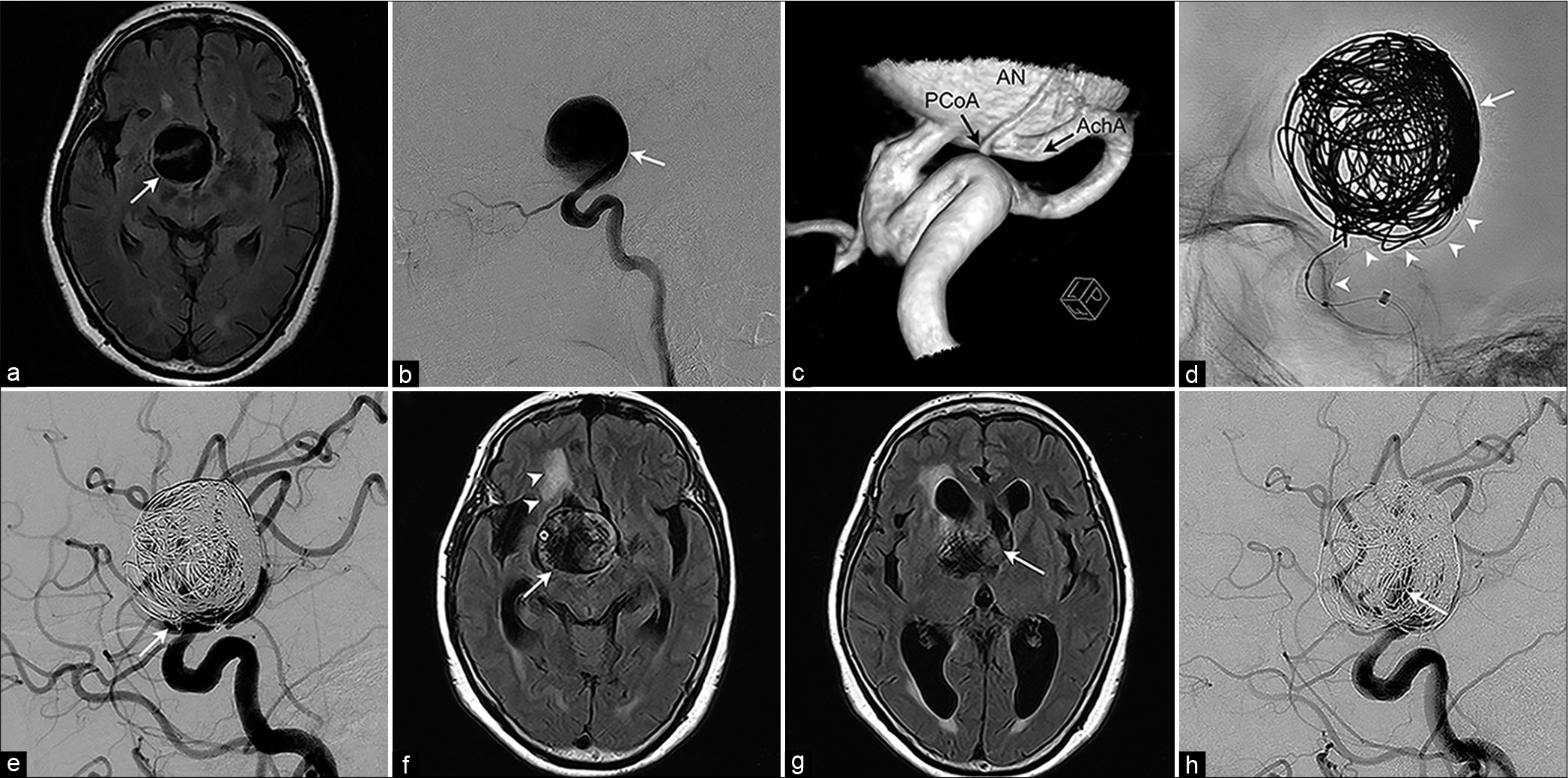
A 74-year-old woman presented with intermittent headache and screening magnetic resonance imaging (MRI) showed a suprasellar mass lesion with a flow void, which was confirmed with cerebral angiography as a 32 mm giant aneurysm at the supraclinoid segment of the right ICA [
At the 6-month follow-up, the patient was neurologically intact and the aneurysm was stable in size. However, she presented with gait disturbance, cognitive dysfunction, and symptomatic seizure, 10 months after FD placement. Repeat MRI showed aneurysmal enlargement to 38 mm with remarkable perianeurysmal edema, together with hydrocephalus caused by obstruction of bilateral foramen of Monro [
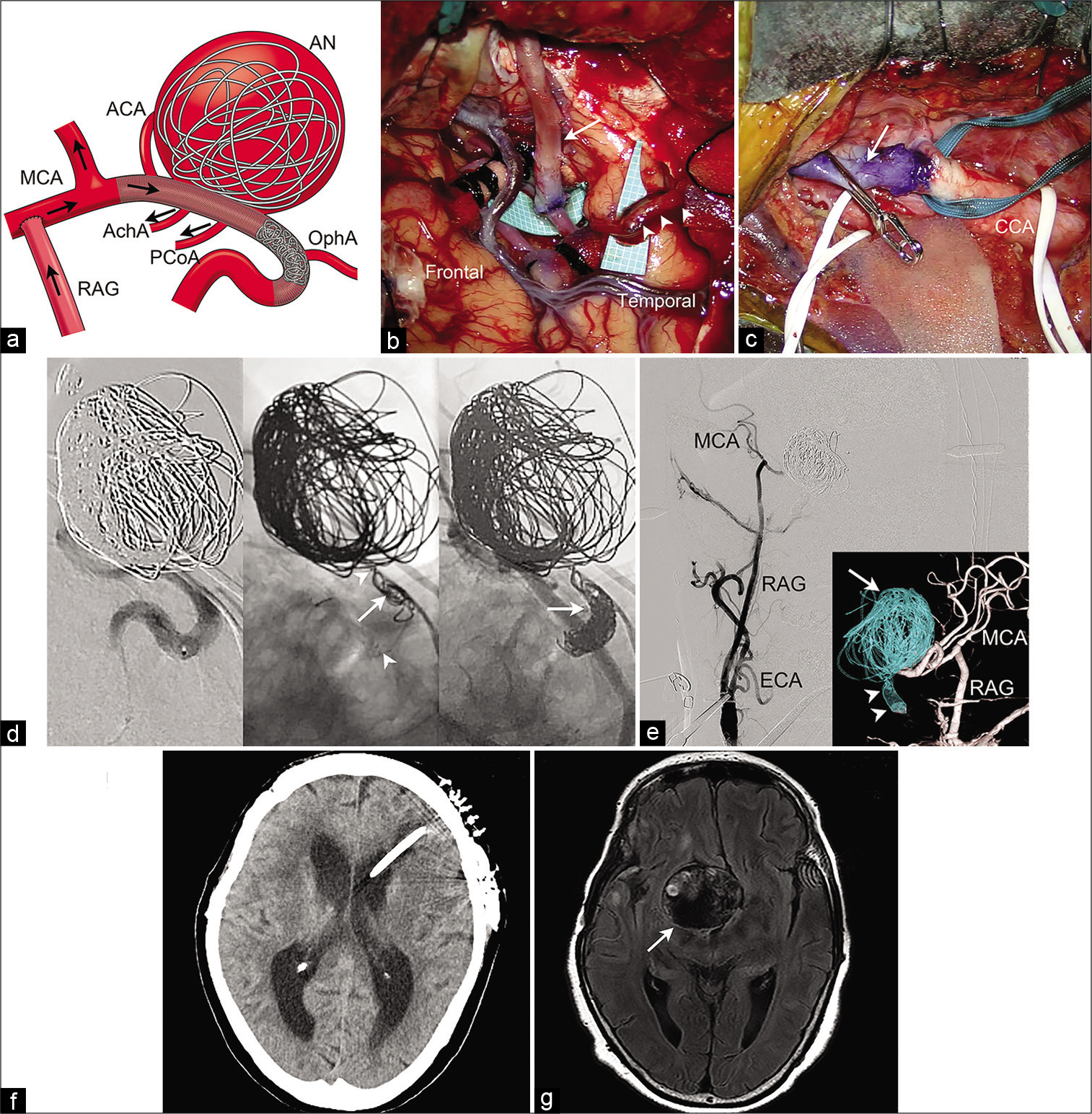
Operation was performed in the hybrid operating room where both surgery and flat-panel detector angiography (Siemens, Berlin, Germany) are available.[
Figure 1:
Radiological imaging of a 74-year-old woman who presented with the right intracranial giant internal carotid artery aneurysm. (a) Head magnetic resonance (MR) imaging shows a 32 mm round mass with a flow void compressing right hypothalamus (arrow). (b) Lateral view of the right internal carotid angiogram shows a giant aneurysm at the supraclinoid portion of the internal carotid artery (arrow). (c) A posterior-lateral-inferior view of reconstructed three-dimensional right internal carotid angiogram shows orifices of the posterior communicating artery and anterior choroidal artery across the wide aneurysmal neck. (d) Intraoperative working angle fluoroscopy shows deployed flow diverter (arrowheads) with intra-aneurysmal detachable coils (arrow) being packed. (e) Postprocedural common carotid angiogram shows remarkable flow reduction of the aneurysm with a small amount of neck remnant (arrow). (f) Head MR imaging 10 months after the flow diverter placement shows aneurysm enlargement to 38 mm (arrow) with perifocal edema (arrowheads). (g) Head MR imaging at the level of the anterior horn of the lateral ventricle shows hydrocephalus caused by obstruction of bilateral foramen of Monro (arrow). (h) The right internal carotid angiogram at aneurysmal enlargement shows residual intra-aneurysmal flow (arrow) with deformed coils.
Figure 2:
Schematic imaging, operative findings, and postoperative course, of the flow alteration treatment in the hybrid operating room. (a) Schematic imaging of the treatment strategy. A high-flow external carotid artery-radial artery graft-middle cerebral artery bypass is made, and the internal carotid artery is obliterated with coils just proximal to the posterior communicating artery. Black arrows show direction of the blood flow after the procedure. (b) Intraoperative photograph of the extracranial-intracranial bypass, cranial part. The free radial artery graft (arrow) and superficial temporal artery (arrowheads) are anastomosed with M2 and M4 portions of the middle cerebral artery, respectively. (c) Intraoperative photograph of the bypass, cervical part. The proximal end of the radial artery graft (arrow) is anastomosed with the external carotid artery. (d) Endovascular procedure. (Left) The course of the right internal carotid artery is shown through contrast medium injection from the microcatheter. (Center) Fluoroscopic imaging shows that an open-cell stent Neuroform Atlas has been deployed between markers at the both ends (arrowheads) and the first coil (arrow) is readily stabilized, being anchored by stent strut. (Right) Fluoroscopy shows that the ophthalmic segment of the right internal carotid artery is completely obliterated with coils (arrow). (e) Conventional anteroposterior and reconstructed posteroanterior view (inset) of the right common carotid angiogram show disappearance of intra-aneurysmal flow (arrow) because of parent artery occlusion with coils (arrowheads), as well as patency of the high-flow bypass. (f) Postoperative head CT scan shows that the patient’s hydrocephalus improves due to the left ventriculoperitoneal shunt placement. (g) Head magnetic resonance imaging 12 months after the flow alteration therapy shows that the aneurysm is stable (arrow) with reduced perifocal edema. ACA: Anterior cerebral artery, AChA: Anterior choroidal artery, AN: Aneurysm, ECA: External carotid artery, MCA: Middle cerebral artery, OphA: Ophthalmic artery, PCoA: Posterior communicating artery, RAG: Radial artery graft.
The patient’s postoperative course was uneventful. Postoperative MRI showed no ischemic complications. She underwent ventriculoperitoneal shunt with endoscopic septostomy 3 weeks after the aneurysm treatment [
DISCUSSION
Recently, FD placement for intractable intracranial aneurysms has been developed. FD treatment reserves anterograde flow of the parent artery and maintains blood flow of the adjacent perforating arteries through the stent strut, while reducing aneurysmal inflow and causing progressive aneurysmal thrombosis.[
Our patient experienced symptomatic aneurysmal growth between 6 and 10 months after FD placement. Because symptomatic growth of giant intracranial aneurysms has a high risk of rupture and has been considered as impending rupture status, immediate treatment to exclude the aneurysm from the normal blood circulation has been proposed.[
This is also the case with aneurysms after FD placement, where extensive intra-aneurysmal thrombogenesis as a part of healing process may even cause thrombus-induced wall degradation depending on hemodynamic conditions, causing aneurysmal rupture.[
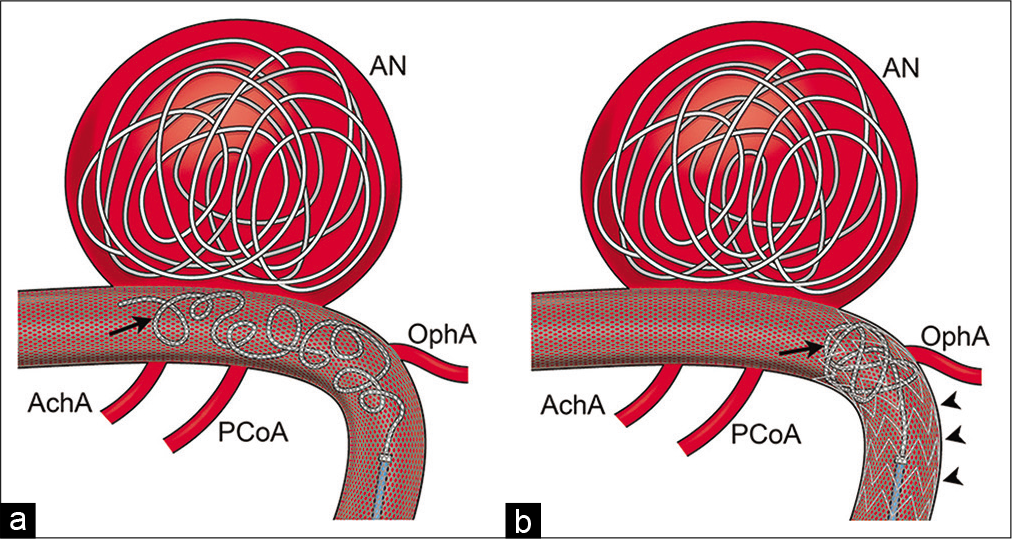
Parent artery occlusion strategy in the present case should be optimized according to anatomical features of the aneurysm. After FD placement, the presence of dense mesh stent in the ICA made clip ligation impossible. In addition, the orifice of AchA was located at the same segment as the aneurysm across the ICA. Complete occlusion of the ICA underneath the entire aneurysmal neck (i.e., internal trapping) was unavailable; thus, proximal occlusion with coils was performed. With this, we sought to reverse and minimize blood flow of the ICA while avoiding ischemic complications.[
Figure 3:
(a) Schematic imaging of coil behavior when the additional Neuroform Atlas stent is absent. The distal coil end (arrow) is unstable and protrudes toward the anterior choroidal artery and the posterior communicating artery in the cylinder-like slippery flow diverter. (b) Schematic imaging of coil behavior when the additional Neuroform Atlas stent (arrowheads) is present. The open-cell struts of Neuroform Atlas works as a scaffold and prevent coils (arrow) from protruding distally.
To the best of our knowledge, this is the first report describing successful tailor-made flow alteration for impending rupture of the giant supraclinoid intracranial aneurysm after FD placement. Although we believe that flow alteration strategy is justified to obtain maximal effect to control intractable aneurysm presenting aggressive feature even after FD placement, little is known about hemodynamic compromise of AchA that has already been covered by FD and subsequently exposed to decreased ICA flow by flow alteration. In our case, outlet flow through the tiny PCoA may be high enough to prevent blind array formation and delayed thrombosis of the AchA. Another possibility is that AchA is intrinsically tolerable for FD coverage as described in the previous literature.[
CONCLUSION
In this article, we described a successful flow alteration therapy for a giant supraclinoid ICA aneurysm enlarging after FD placement. Impending rupture of the FD-treated aneurysms may be safely controlled with tailor-made strategy using extracranial-intracranial bypass and targeted endovascular parent artery occlusion.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abla AA, Zaidi HA, Crowley RW, Britz GW, McDougall CG, Albuquerque FC. Optic chiasm compression from mass effect and thrombus formation following unsuccessful treatment of a giant supraclinoid ICA aneurysm with the pipeline device: Open surgical bailout with STA-MCA bypass and parent vessel occlusion. J Neurosurg Pediatr. 2014. 14: 31-7
2. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013. 267: 858-68
3. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke. 2013. 44: 442-7
4. Cagnazzo F, Mantilla D, Rouchaud A, Brinjikji W, Lefevre PH, Dargazanli C. Endovascular treatment of very large and giant intracranial aneurysms: Comparison between reconstructive and deconstructive techniques-a meta-analysis. AJNR Am J Neuroradiol. 2018. 39: 852-8
5. Fukuda H, Yanagawa T, Horikawa F, Nakajima N, Kitagawa M, Lo B. “Clip anchor-assisted coil embolization” for endovascular parent artery occlusion of intracranial traumatic aneurysm. J Stroke Cerebrovasc Dis. 2019. 28: 104374
6. Goertz L, Hesse N, Liebig T, Ahmad W, Abdullayev N, Krischek B. Retreatment strategies for recurrent and residual aneurysms after treatment with flow-diverter devices. Neuroradiology. 2020. 62: 1019-28
7. Hasegawa H, Inoue T, Tamura A, Saito I. Tailored flow sequestration treatment using high-flow and low-flow bypass for partially thrombosed giant internal carotid artery aneurysm-a technical case report. Neurosurg Rev. 2016. 39: 699-705
8. Iihara K, Satow T, Matsushige T, Kataoka H, Nakajima N, Fukuda K. Hybrid operating room for the treatment of complex neurovascular and brachiocephalic lesions. J Stroke Cerebrovasc Dis. 2013. 22: e277-85
9. Kado K, Hirai S, Kobayashi S, Kobayashi E, Yamakami I, Uchino Y. Potential role of the anterior spinal artery in preventing propagation of thrombus in a therapeutically occluded vertebral artery: Angiographic studies before and after endovascular treatment. Neuroradiology. 2002. 44: 347-54
10. Kazumata K, Nakayama N, Nakamura T, Kamiyama H, Terasaka S, Houkin K. Changing treatment strategy from clipping to radial artery graft bypass and parent artery sacrifice in patients with ruptured blister-like internal carotid artery aneurysms. Neurosurgery. 2014. 10: 66-72
11. Kulcsár Z, Houdart E, Bonafé A, Parker G, Millar J, Goddard AJ. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol. 2011. 32: 20-5
12. Matsukawa H, Miyata S, Tsuboi T, Noda K, Ota N, Takahashi O. Rationale for graft selection in patients with complex internal carotid artery aneurysms treated with extracranial to intracranial high-flow bypass and therapeutic internal carotid artery occlusion. J Neurosurg. 2018. 128: 1753-61
13. Murakami K, Shimizu H, Matsumoto Y, Tominaga T. Acute ischemic complications after therapeutic parent artery occlusion with revascularization for complex internal carotid artery aneurysms. Surg Neurol. 2009. 71: 434-41
14. Oishi H, Teranishi K, Yatomi K, Fujii T, Yamamoto M, Arai H. Flow diverter therapy using a pipeline embolization device for 100 unruptured large and giant internal carotid artery aneurysms in a single center in a Japanese population. Neurol Med Chir (Tokyo). 2018. 58: 461-7
15. Rangel-Castilla L, Munich SA, Jaleel N, Cress MC, Krishna C, Sonig A. Patency of anterior circulation branch vessels after Pipeline embolization: Longer-term results from 82 aneurysm cases. J Neurosurg. 2017. 126: 1064-9
16. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: A systematic review. Stroke. 1998. 29: 251-6
17. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10