- Division of Neurosurgery, ARNAS Civico Hospital, Palermo, Italy
- Department of Experimental Biomedicine and Clinical Neurosciences, School of Medicine, Neurosurgical Clinic, AOUP “Paolo Giaccone”, Palermo, Italy
Correspondence Address:
Alessandro Villa
Department of Experimental Biomedicine and Clinical Neurosciences, School of Medicine, Neurosurgical Clinic, AOUP “Paolo Giaccone”, Palermo, Italy
DOI:10.4103/sni.sni_89_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Natale Francaviglia, Domenico Gerardo Iacopino, Gabriele Costantino, Alessandro Villa, Pietro Impallaria, Francesco Meli, Rosario Maugeri. Fluorescein for resection of high-grade gliomas: A safety study control in a single center and review of the literature. 11-Jul-2017;8:145
How to cite this URL: Natale Francaviglia, Domenico Gerardo Iacopino, Gabriele Costantino, Alessandro Villa, Pietro Impallaria, Francesco Meli, Rosario Maugeri. Fluorescein for resection of high-grade gliomas: A safety study control in a single center and review of the literature. 11-Jul-2017;8:145. Available from: http://surgicalneurologyint.com/surgicalint-articles/fluorescein-for-resection-of-high%e2%80%91grade-gliomas-a-safety-study-control-in-a-single-center-and-review-of-the-literature/
Abstract
Background:The importance of a complete resection of high-grade gliomas (HGGs) has been highlighted in scientific literature, in order to limit tumor recurrence and above all to improve disease-free survival rates. Several fluorescent biomarkers have been tested to improve intraoperative identification of residual tumor; 5-aminolevulinic acid (5-ALA) and fluorescein sodium (FS) are now starting to play a central role in glioma surgery. We performed a retrospective analysis on 47 patients operated for HGGs. Here we report our preliminary data.
Methods:Data of 47 consecutive patients with HGG have been collected in our study (25 males, 22 females; mean age: 60.3 years, range: 27–86 years). Fluorescein (5 mg/kg of body weight) was injected intravenously right after the induction of general anesthesia. A YELLOW 560 filter was used on an OPMI Pentero 900 microscope (Carl Zeiss Meditec, Oberkochen, Germany) to complete a microsurgical tumor removal. Glioma resection and quality of life were evaluated preoperative and postoperatively.
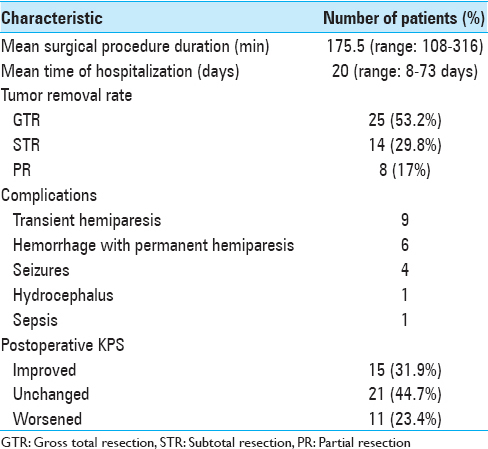
Results:Gross total resection (GTR) was achieved in 53.2% (n = 25) of patients. A subtotal resection (STR) (>95%) was achieved in 29.8% (n = 14), while a partial resection (PR) (n = 8) of patients. Overall, in 83% (n = 39) of patients who underwent fluorescence-guided surgery the resection rate achieved was >95%. No adverse effects correlated to fluorescein have been recorded.
Conclusion:Fluorescein seems to be safe and effective in the resection of HGGs, allowing a high rate of gross total removal of contrast enhanced areas.
Keywords: 5-aminolevulinic acid, extent of resection, fluorescein sodium, high-grade gliomas, YELLOW 560 filter
INTRODUCTION
Gliomas are the most common primary malignant brain tumors in adults, nearly represent 80%, with poor prognosis in their high-grade forms.[
MATERIALS AND METHODS
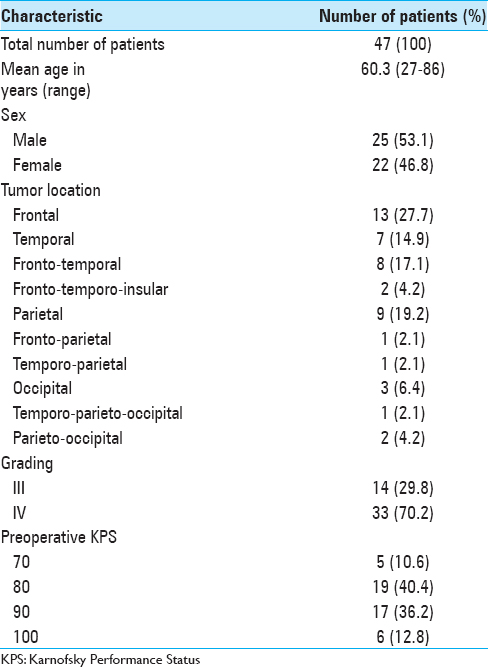
In this retrospective study, after the approval of Local Ethic Committee, we collected data of 47 patients (25 males, 22 females; mean age: 60.3 years, range: 27–86 years). All patients had been surgically treated at the Neurosurgical Unit of ARNAS Civico Hospital, between September 2015 and November 2016 [
Surgical protocol
In all cases 5 mg/kg body weight of FS was injected intravenously via the central venous line, after the induction of anesthesia. Vital signs were monitored for 15 min. Under white light, no fluorescent effect was detected. The fluorescent dye was visible under the YELLOW 560 nm filter, on the OPMI Pentero 900 surgical microscope (Carl Zeiss Meditec, Oberkochen, Germany). The fluorescence shaped the vital tumor margins [
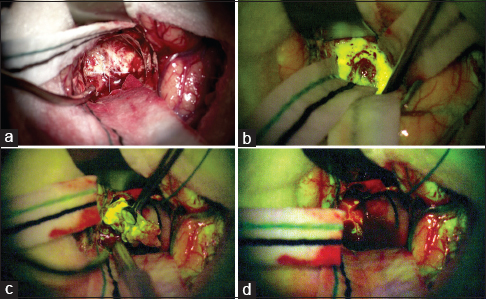
Figure 1
Intraoperative view of a right frontal glioblastoma under normal white xenon-light illumination (a) and at the beginning of tumor removal under the YELLOW 560 nm filter (b). During tumor removal on the Pentero 900 surgical microscope, there is a clear delineation of the tumor area which shows significant fluorescein sodium enhancement revealing the boundary between the bright yellow tumor and the surrounding brain (c); at the end of the removal, no residual tumor tissue is evident (d)
Pre- and postoperative clinical assessment
Each patient general physical performance was recorded using the KPS. The median preoperative KPS score was 85.1 (range: 70–100). Preoperative clinical evaluation was performed at the admission to the neurosurgical unit. A second evaluation was conducted during the postoperative course, on discharge and at the first outpatient clinic visit approximately 1 month later. The neurological exam was evaluated by a neurologist. Surgery was followed by radiotherapy with concomitant and adjuvant temozolomide in 85.1% of cases, according to the Stupp protocol.[
Radiological assessment
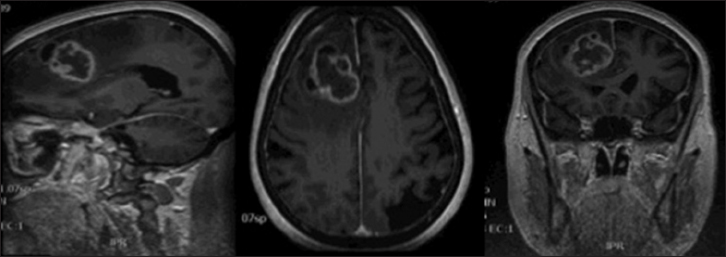
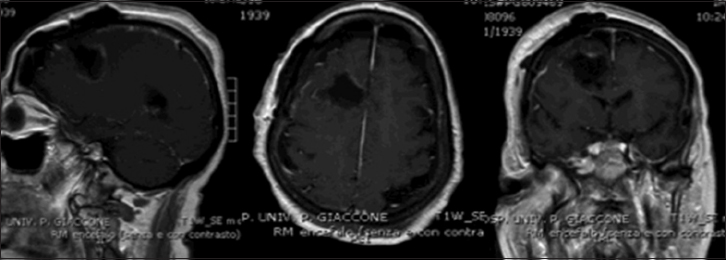
Postoperatively, the extent of tumor resection was identified by 35 contrast enhanced T1 weighted MRI and 12 contrast enhanced computed tomography (CT scan), 72 h after surgery. Three categories were distinguished: no residual tumor tissue = gross total resection (GTR); minimal residual tumor tissue = subtotal resection (STR) and partial resection (PR). GTR was defined as resection where no residual enhanced tumor is visible, STR was defined as nearly total (>95%), PR as <95%. Postoperative tumor volumes after surgery were calculated using an open-source, free medical image viewer software (OsiriX for Mac) on enhanced residual tissue (in T1 weighted MRI or CT scan) [Figures
Histological examination
Histological analyses were performed with standard procedures. The classification was conducted on the basis of the current WHO classification of tumors of the central nervous system.
RESULTS
The average duration of the surgical procedure (“skin to skin”) was 175.5 min (range: 108–316 min); the median length of hospital stay was 20 days (range: 8–73 days). Patients and tumor data and results are summarized in Tables
DISCUSSION
The importance of radical resection in glioma surgery has been already stressed in literature. Although rare cases of spontaneous regression of benign tumors after incomplete removal are described,[
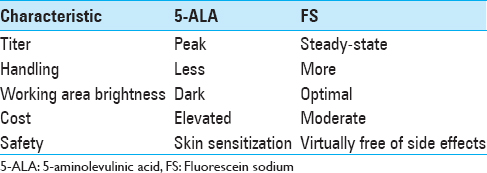
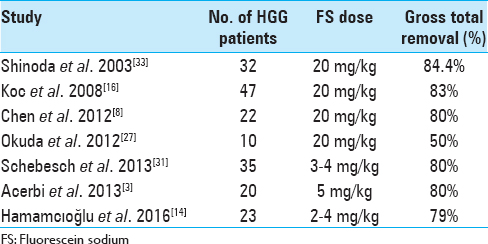
In our experience, the use of FS together with YELLOW 560 nm filter has been found “helpful” in tumor removal in all the surgical procedures, allowing a better visual discrimination between the pinkish brain tissue and the yellow stained tumor tissue. This also seemed to be more comfortable to the surgeon eyes. The enhancement of tumoral tissue, which corresponds to the contrast enhancement of preoperative MRI, was visible immediately after dural opening, usually 30 min after the FS administration, and lasted until the end of tumor removal. The rate of tumor removal in our study was similar to that of previous studies. GTR was achieved in 53.2% (n = 25) of patients. A STR (>95%) was achieved in 29.8% (n = 14) of them, while a PR (<95%) was obtained in 17% (n = 8) of patients. Globally, a resection >95% was achieved in 83% (n = 39) of patients who underwent fluorescence-guided surgery. Moreover, FS seemed to be safe and effective in the intraoperative visual phase by distinguishing tumor from normal brain tissue and in the postoperative neuroimaging control. The resection was also maximized with the aid of neuronavigation system in eloquent areas, trying to avoid additional neurological deficit to the patient. The median postoperative KPS score was 83.4 (range: 50–100). Comparing pre- and postoperative scores, the latter was found higher in 15 patients, lower in 11, and stable in 21 patients. As in Schebesch, Acerbi, and Hamamcıoğlu series, we also report the use of low dose FS about 2 mL (5 mg/kg body weight). This low dose, together with YELLOW 560 nm filter, allowed an easier intraoperative management than higher doses of FS or 5-ALA, without the need to wait for the dye peak.[
CONCLUSIONS
The intraoperative identification and resection of HGGs is a significant and important challenge in neurosurgery. In our series fluorescence-guided surgery of HGGs using FS has been a good tool in achieving GTR and in distinguishing tumoral by normal brain tissue. Larger-scale studies are now needed, to quantify the efficacy of fluorescein-guided surgery in improving the extent of resection as well as the progression-free and overall patient survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
No financial support was received. The manuscript or parts of it are not under consideration by another journal or electronic publication and have not been previously published or presented at a meeting. The authors have no proprietary or commercial interest in any materials or method discussed in this article.
References
1. Acerbi F, Broggi M, Broggi G, Ferroli P. What is the best timing for fluorescein injection during surgical removal of high-grade gliomas?. Acta Neurochir. 2015. 157: 1377-8
2. Acerbi F, Broggi M, Eoli M, Anghileri E, Cavallo C, Boffano C. Is fluorescein-guided technique able to help in resection of high-grade gliomas?. Neurosurg Focus. 2014. 36: E5-
3. Acerbi F, Broggi M, Eoli M, Anghileri E, Cuppini L, Pollo B. Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: Preliminary results in 12 cases. Acta Neurochir. 2013. 155: 1277-86
4. Acerbi F, Cavallo C, Broggi M, Cordella R, Anghileri E, Eoli M. Fluorescein-guided surgery for malignant gliomas: A review. Neurosurg Rev. 2014. 37: 547-57
5. Barbagallo GM, Certo F, Caltabiano R, Chiaramonte I, Albanese V, Visocchi M. Role of intraoperative indocyanine green video-angiography to identify small, posterior fossa arteriovenous malformations mimicking cavernous angiomas. Technical report and review of the literature on common features of these cerebral vascular malformations. Clini Neurol Neurosurg. 2015. 138: 45-51
6. Barbagallo GMV, Palmucci S, Visocchi M, Paratore S, Attinà G, Sortino G. Portable Intraoperative Computed Tomography Scan in Image-Guided Surgery for Brain High-grade Gliomas: Analysis of Technical Feasibility and Impact on Extent of Tumor Resection. Oper Neurosurg. 2016. 12: 19-30
7. Berger MS. The fluorescein-guided technique. Neurosurgical Focus. 2014. 36: E6-
8. Chen B, Wang H, Ge P, Zhao J, Li W, Gu H. Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int J Med Sci. 2012. 9: 708-14
9. Colditz MJ, Jeffree RL. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: Clinical, radiological and pathological studies. J Clin Neurosci. 2012. 19: 1471-4
10. Diaz RJ, Dios RR, Hattab EM, Burrell K, Rakopoulos P, Sabha N. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J Neurosurg. 2015. 122: 1360-9
11. Dilek O, Ihsan A, Tulay H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J Clin Neurosci. 2011. 18: 430-1
12. Graziano F, Certo F, Basile L, Maugeri R, Grasso G, Meccio F. Autologous fibrin sealant (Vivostat((R))) in the neurosurgical practice: Part I: Intracranial surgical procedure. Surg Neurol Int. 2015. 6: 77-
13. Graziano F, Maugeri R, Basile L, Meccio F, Iacopino DG. Aulogous fibrin sealant (Vivostat((R))) in the neurosurgical practice: Part II: Vertebro-spinal procedures. Surg Neurol Int. 2016. 7: S77-82
14. Hamamcioglu MK, Akcakaya MO, Goker B, Kasimcan MO, Kiris T. The use of the YELLOW 560 nm surgical microscope filter for sodium fluorescein-guided resection of brain tumors: Our preliminary results in a series of 28 patients. Clin Neurol Neurosurg. 2016. 143: 39-45
15. Hernandez-Gonzalez G, Marchione P, De Angelis F, Giannone C, Kouleridou A, Spallone A. Long-term survival in cerebellar glioblastoma multiforme. Case report. J Neurosurg Sci. 2012. 56: 379-81
16. Koc K, Anik I, Cabuk B, Ceylan S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br J Neurosurg. 2008. 22: 99-103
17. Kuroiwa T, Kajimoto Y, Ohta T. Development of a fluorescein operative microscope for use during malignant glioma surgery: A technical note and preliminary report. Surg Neurol. 1998. 50: 41-
18. La Torre D, Maugeri R, Angileri FF, Pezzino G, Conti A, Cardali SM. Human leukocyte antigen frequency in human high-grade gliomas: A case-control study in Sicily. Neurosurgery. 2009. 64: 1082-
19. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
20. Li Y, Rey-Dios R, Roberts DW, Valdes PA, Cohen-Gadol AA. Intraoperative fluorescence-guided resection of high-grade gliomas: A comparison of the present techniques and evolution of future strategies. World Neurosurg. 2014. 82: 175-85
21. Martirosyan NL, Georges J, Eschbacher JM, Cavalcanti DD, Elhadi AM, Abdelwahab MG. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg Focus. 2014. 36: E16-
22. Maugeri R, Basile L, Giugno A, Graziano F, Iacopino DG. Impasse in the management of recurrent basal cell carcinoma of the skull with sagittal sinus erosion. Interdisc Neurosurg. 2015. 2: 160-3
23. Maugeri R, Giammalva GR, Graziano F, Iacopino DG. May Autologue Fibrin Glue Alone Enhance Ossification? An Unexpected Spinal Fusion. World Neurosurg. 2016. 95: 611-2
24. Maugeri R, Schiera G, Di Liegro CM, Fricano A, Iacopino DG, Di Liegro I. Aquaporins and Brain Tumors. Int J Mol Sci. 2016. 17:
25. Moore GE, Peyton WT. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg. 1948. 5: 392-8
26. Murray KJ. Improved surgical resection of human brain tumors: Part I. A preliminary study. Surg Neurol. 1982. 17: 316-9
27. Okuda T, Yoshioka H, Kato A. Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters. J Clin Neurosci. 2012. 19: 1719-22
28. Rey-Dios R, Hattab EM, Cohen-Gadol AA. Use of intraoperative fluorescein sodium fluorescence to improve the accuracy of tissue diagnosis during stereotactic needle biopsy of high-grade gliomas. Acta Neurochir. 2014. 156: 1071-5
29. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
30. Schebesch KM, Hoehne J, Hohenberger C, Proescholdt M, Riemenschneider MJ, Wendl C. Fluorescein sodium-guided resection of cerebral metastases-experience with the first 30 patients. Acta Neurochir. 2015. 157: 899-904
31. Schebesch KM, Proescholdt M, Hohne J, Hohenberger C, Hansen E, Riemenschneider MJ. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery-a feasibility study. Acta Neurochir. 2013. 155: 693-9
32. Schwake M, Stummer W, Suero Molina EJ, Wolfer J. Simultaneous fluorescein sodium and 5-ALA in fluorescence-guided glioma surgery. Acta Neurochir. 2015. 157: 877-9
33. Shinoda J, Yano H, Yoshimura S, Okumura A, Kaku Y, Iwama T. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg. 2003. 99: 597-603
34. Smith LG, Nakano I. Fluorescence-guided brain tumor surgery. World Neurosurg. 2012. 78: 559-64
35. Spallone A, Visocchi M, M DIC, Belvisi D. Subependymoma of septum pellucidum presenting with cough and exertional headache: A case report of spontaneous regression after incomplete surgical removal. J Neurosurg Sci. 2016. 60: 283-4
36. Stummer W, Meinel T, Ewelt C, Martus P, Jakobs O, Felsberg J. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neuro-oncol. 2012. 108: 89-97
37. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
38. Su X, Huang QF, Chen HL, Chen J. Fluorescence-guided resection of high-grade gliomas: A systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2014. 11: 451-8