- Department of Neurosurgery, Great Ormond Street Hospital for Children, London, United Kingdom.
Correspondence Address:
Zubair Tahir, Department of Neurosurgery, Great Ormond Street Hospital for Children, London, United Kingdom.
DOI:10.25259/SNI_419_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Saurav Samantray, Adikarige Haritha Dulanka Silva, Alexandra Valetopoulou, Zubair Tahir. Foramen magnum decompression with cervical syringotomy for Chiari malformation type I with syringomyelia – A useful adjunct in selected cases. 22-Sep-2023;14:341
How to cite this URL: Saurav Samantray, Adikarige Haritha Dulanka Silva, Alexandra Valetopoulou, Zubair Tahir. Foramen magnum decompression with cervical syringotomy for Chiari malformation type I with syringomyelia – A useful adjunct in selected cases. 22-Sep-2023;14:341. Available from: https://surgicalneurologyint.com/surgicalint-articles/12568/
Abstract
Background: Persistent or worsening syringomyelia after foramen magnum decompression (FMD) for Chiari I malformation (CIM) can be challenging to manage. We present a previously unpublished surgical technique of FMD with concomitant cervical syringotomy in selected patients.
Methods: A retrospective analysis of prospectively collected data was carried out. Patients who underwent FMD and expansion duraplasty (FMDD) with concomitant syringotomy were collected.
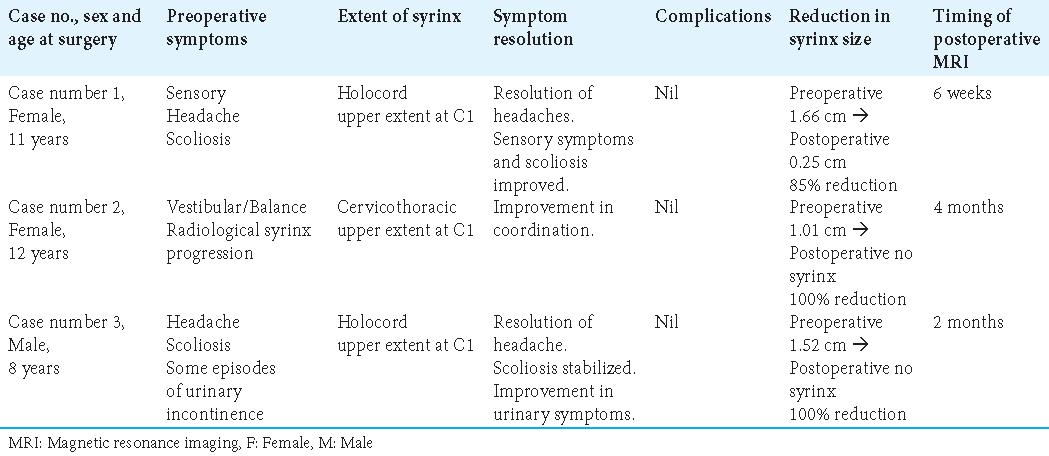
Results: Three patients with CIM with high cervical syringomyelia who underwent FMDD with concurrent syringotomy were identified. All cases had an idiopathic CIM. Improvement in clinical symptoms was noticed in all patients. Early postoperative imaging (within 6 weeks–4 months) showed syrinx transverse diameter reduction in the range of 85–100%. There were no postoperative complications.
Conclusion: FMDD with concurrent high cervical syringotomy through a standard approach in selected cases of CIM with high cervical syringes achieves clinical improvement without additional complications.
Keywords: Chiari malformation, Foramen magnum decompression, Syringomyelia, Syringotomy
INTRODUCTION
The surgical practice to manage hindbrain herniation has evolved over the past several decades. Chiari I malformation (CIM), first described in 1891, involves caudal descent of the cerebellar tonsils through the foramen magnum. The association between CIM and syringomyelia is seen in 35–75% of the cases.[
While most neurosurgeons agree that foramen magnum decompression (FMD) is the mainstay of surgical treatment, there is considerable diversity of opinion regarding the extent of surgery with a wide range of permutations of operative technique.[
FMD with expansion duraplasty (FMDD) on its own successfully addresses cerebrospinal fluid (CSF) flow dynamics at the craniocervical junction thereby affecting the syrinx filling mechanism concurrently. Although other techniques have also been utilized to try to improve the rapidity of decompressing the syrinx, such as concurrent FMD with syringo-subarachnoid shunt described by Sgouros and Williams.[
We, therefore, considered whether, in a highly selected group of patients with CIM and high, tense cervical and medullary syringomyelia, the incorporation of syringotomy during FMDD could achieve rapid decompression and drainage of syrinx without any added risks and complications.
MATERIALS AND METHODS
We present three patients with CIM with extensive syringomyelia with the upper part of the syrinx reaching up to the cervicomedullary junction, who underwent FMDD with concurrent syringotomy. All cases had an idiopathic CIM with no associated craniovertebral junction anomaly/instability or hydrocephalus. The decision for concurrent syringotomy for all patients was taken preoperatively based on the location of the syrinx and after review of imaging confirmed an extensive, tense syrinx accessible through our routine surgical exposure for FMD (suboccipital craniectomy and C1 posterior arch laminectomy). All patients included in this study were consented preoperatively regarding the syringotomy.
The predominant outcome measures were improvement in symptomatology and improvement in syrinx status on magnetic resonance imaging (MRI). The presence or absence of surgical complications was also noted. The radiological features of the syringes were assessed using comparative measurements. A collapse meant that the diameter of the syrinx had become zero. This could involve a segment of the syrinx (segmental collapse) or the whole syrinx (total collapse). In syringes or segments without collapse, we simply measured the percentage change in the maximum transverse diameter of the syrinx.
The Institutional Review Board/Ethics Committee approval and patient consent were not sought, as this was a retrospective study.
Surgical technique
First, we describe our standard technique for conducting FMD with duraplasty (FMDD). Intraoperative neurophysiological monitoring is used in all cases with transcranial motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs). The midline skin incision was followed by the musculofascial flap elevated in a T-shaped fashion and subperiosteal dissection to expose the subocciput, foramen magnum, and posterior arch of C1. Suboccipital craniectomy at the foramen magnum (3 cm width × 2 cm height) is performed, followed by C1 posterior arch laminectomy. Extradural constrictive bands including the posterior atlanto-occipital membrane are excised followed by a Y-shape dural opening and the tonsils are visualized.
Depending on the extent of tonsillar herniation, under magnification, the cerebellar tonsils are reduced with bipolar coagulation and expose the Obex. The Obex and foramen of Magendie are explored to treat any obstruction to CSF outflow from the fourth ventricle [
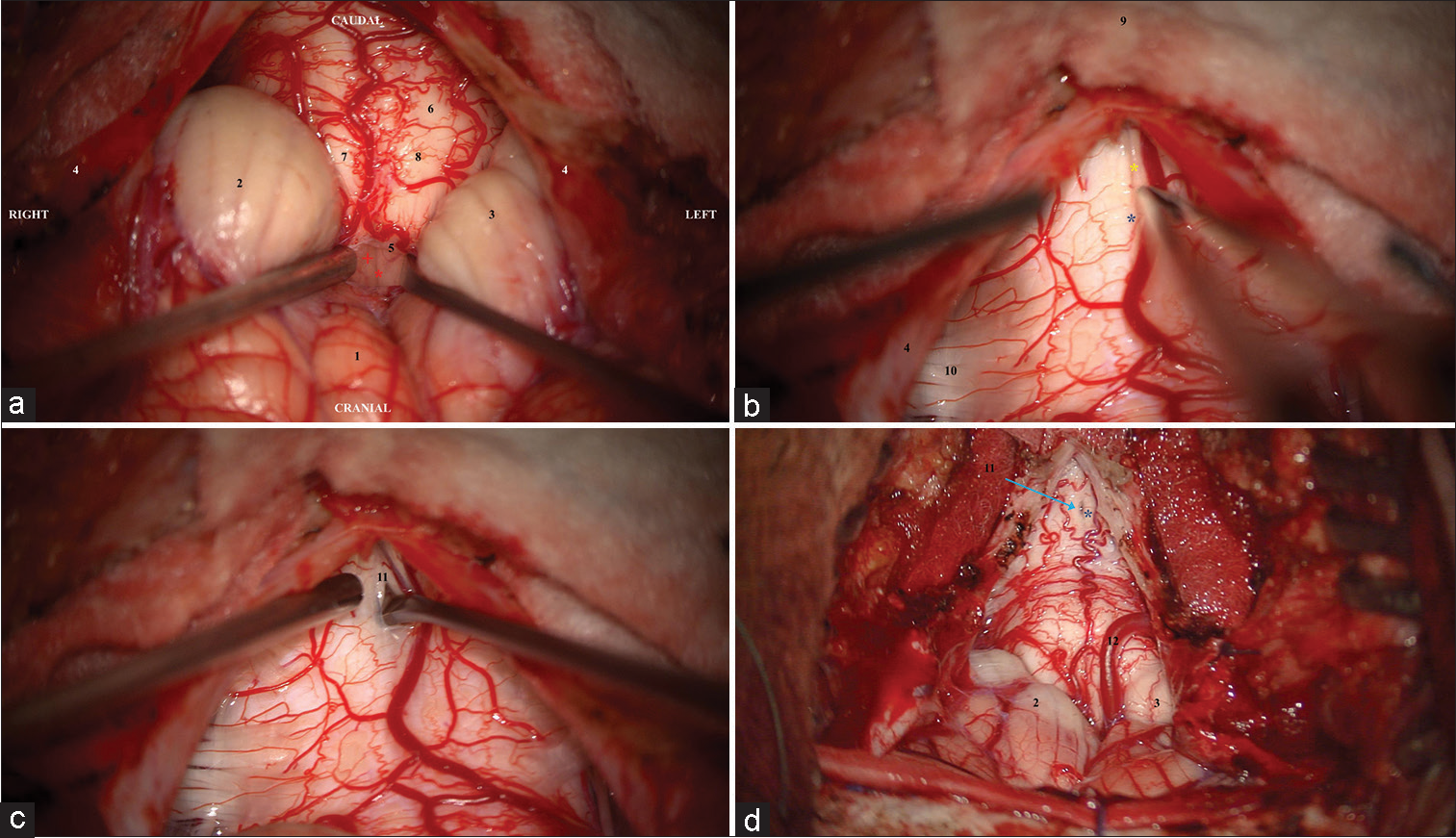
Figure 1:
Intraoperative microscopic photographs following foramen magnum decompression, C1 laminectomy, and y-shaped dural and arachnoid opening to demonstrate sequence of steps for syringotomy. (a) (1) Inferior vermis, (2) right and (3) left cerebellar tonsils coagulated to superiorly retract, (4) right and left dural flaps reflected, (5) arachnoid veil obstructing 4th ventricular outflow through foramen of Magendie opened exposing Obex with (Red*) median sulcus and (Red +) base of trigones, (6) expanded and tense cervical cord with (7) right and left, and (8) dorsal columns. (b) (4) Right dural flap, (9) spinous process of C2 not exposed is covered by cottonoid, and (10) right dorsal C2 nerve rootlets from the dorsal root entry zone. (Yellow*) Median sulcus of the spinal cord demonstrated. Phase reversal has not been reliable in identifying the midline likely due to fiber stretch due to the expansile syrinx. Careful exploration can demonstrate midline pial vessels dipping into the median sulcus (Blue*). (c) (11) An ophthalmic/diamond blade can be used to make the initial syringotomy which is widened with a micro-dissector through the sulcus. With the syrinx being tense and bulging, only a small opening is needed with rapid drainage of fluid and decompression of the intramedullary cord. The cavity can be further drained using a micro-suction tip. (d) (Blue*) Midline pial vessels dipping into the median sulcus (11 with Blue arrow) The small syringotomy can be visualized with the spinal cord significantly less tense and expanded compared to previously (12). The left posterior inferior cerebellar artery is observed as are the (2 and 3) coagulated cerebellar tonsils.
Intraoperative ultrasound is used to confirm the extent of the syrinx cavity on the upper cervical cord. Often, the midline median sulcus is visible together with an expanded cord. Neurophysiological monitoring and mapping can be used to identify the median sulcus with a phase reversal technique, although this has not been reliable in our short experience, owing most likely to the displacement of fibers due to the expansive syrinx cavity. We have found anatomical markers an excellent and reproducible indicator in particular pial arteries that can be seen dipping inward at the median sulcus on the dorsal spinal cord surface [
A syringotomy is performed using a sharp ophthalmic/ diamond blade and expanded in a craniocaudal fashion using a microdissector [
RESULTS
Case 1
This is an 11-year-old girl with CIM and an extensive holocord syrinx [
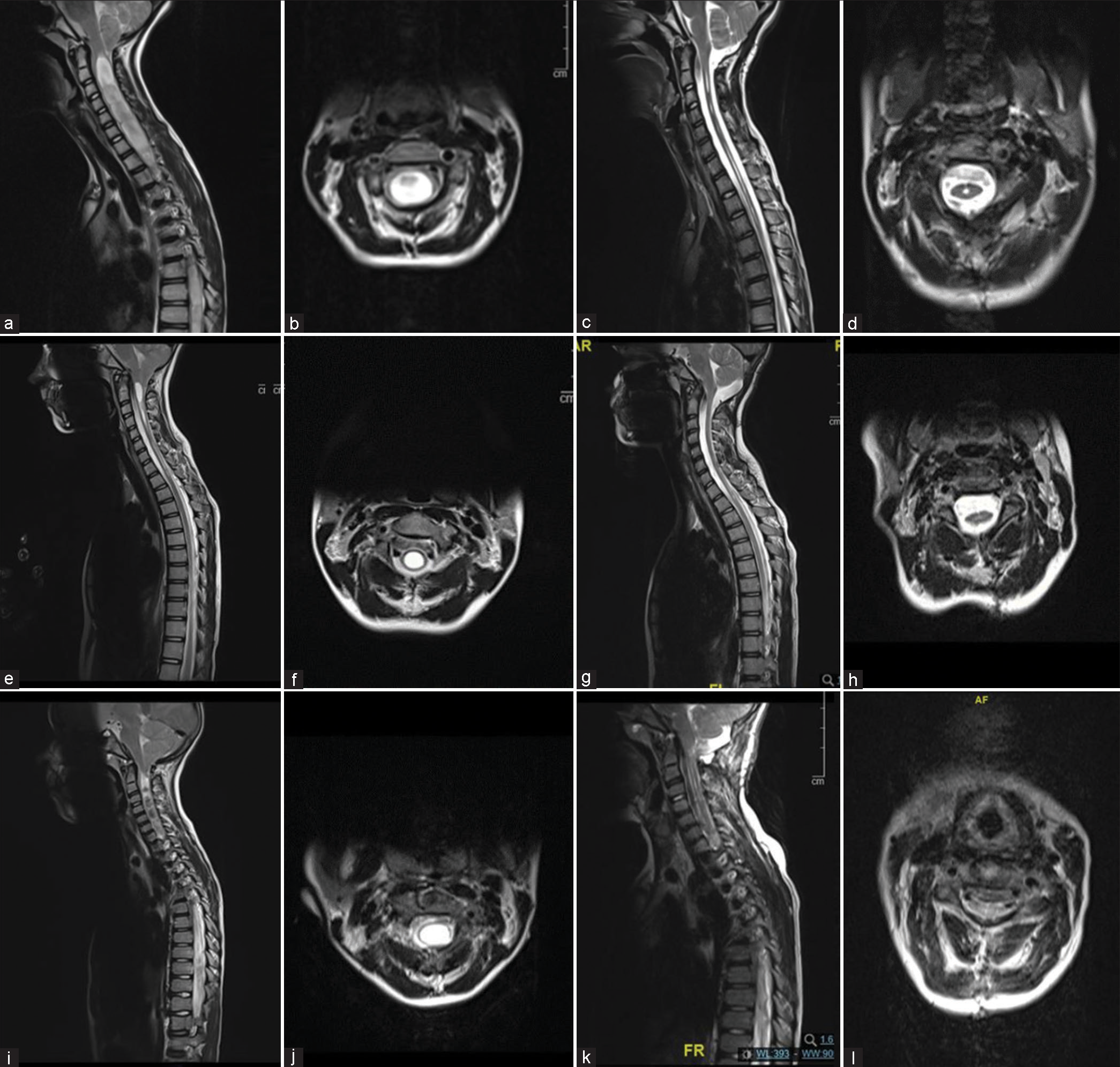
Figure 2:
Pre- and post-operative magnetic resonance imaging T2-weighted images demonstrating impact on syrinx cavity in our three patients. (a and b) Case 1 preoperative sagittal and axial imaging with extensive multiseptated holocord syrinx and evidence of turbulent flow within the syrinx cavity as evidenced by the hypo-intense signal changes within. (c and d) Case 1 postoperative sagittal and axial imaging at 1½ month follow-up confirming significant reduction in all septated cavities. (e and f) Case 2 preoperative sagittal and axial imaging with cervicothoracic syrinx cavity and a thin rim of neural tissue. (g and h) Case 2 postoperative sagittal imaging at 4-month follow-up demonstrating significant resolution. (i and j) Case 3 preoperative sagittal and axial imaging with extensive holocord syrinx and minimal neural tissue overlying the dorsal aspect. (k and l) Case 3 postoperative sagittal imaging at 2-month follow-up demonstrating significant resolution of syrinx.
Tonsillar ascent and syrinx reduction were visible on her first postoperative MRI at 6 weeks. There was a segmental collapse of the syrinx with an 85% change noted in maximum transverse diameter (from 1.66 cm preoperatively to 0.25 cm postoperatively) [
Case 2
This is a 12-year-old girl with CIM and a cervicothoracic syrinx [
Her postoperative MRI 4 months after surgery demonstrated ascent of tonsils, a total collapse of the syrinx, and thus the complete resolution of syrinx from a maximum preoperative transverse diameter of 1.01 cm [
Case 3
This is an 8-year-old boy with CIM and holocord syrinx [
A total collapse of the syrinx was demonstrated on the first postoperative MRI done 2 months after surgery with a 100% reduction from the preoperative maximum transverse diameter of 1.52 cm [
DISCUSSION
FMD is the most common primary surgical procedure used to treat CIM with syringomyelia, the intent being to restore CSF flow dynamics across the foramen magnum thus reversing the pathophysiologic mechanisms leading to syrinx formation. However, FMD is known to fail in improving syringomyelia in a significant number of postoperative cases of CIM, and the persistence of syrinx can have permanent sequelae.[
Surgical options to treat persistent/progressive syrinx after FMD include revision of the FMD and syrinx shunting procedures such as syringo-subarachnoid, syringopleural, and syringoperitoneal shunts.[
Soleman et al. has described concurrent FMD and syringosubarachnoid shunt for CIM and syringomyelia.[
We present a short case series of three patients with CIM and concurrent syringomyelia who underwent FMDD with the addition of a syringotomy to achieve rapid decompression of radiologically expansile and tense syrinx cavities. These patients were selected for syringotomy based on the presence of:
Syrinx extending to the high cervical spine, accessible through our standard exposure for FMD (i.e., following FMD and C1 laminectomy) Expansive syrinx cavity extending to the surface of the dorsal pial spinal cord surface and median sulcus and thereby directly accessible with minimal neural tissue transgression Availability of intraoperative neurophysiological monitoring.
Our aim in the presence of extensive, expansile syrinx cavities in particular with rostral extension toward the medulla was to achieve a rapid decompression of the syrinx with no need for any further incisions. There was clinical improvement in all cases. There were no significant postoperative complications. Radiological improvement was reported in all three cases, with the earliest improvement in the 6th postoperative week. The syrinx transverse diameter reduction was in the range of 85–100%. There was no syrinx recurrence in any of the three cases at their last follow-up. In a study of 85 pediatric patients, the median time to >50% reduction in syrinx anteroposterior diameter was 8 months after FMD for CIM.[
Limitations to our study include small numbers. Furthermore, only selected patients are eligible for consideration, and it is not suitable for patients in whom the syrinx is below C2 level and thus not accessible through standard exposure. We feel that our case series demonstrates that syringotomy can be safely performed in selected patients with early resolution of syrinx in all patients.
CONCLUSION
FMDD with concurrent syringotomy in selected cases of CIM with high cervical syringes achieves surgical objectives in a single exposure. This procedure results in clinical improvement with no additional complications. Prolonged follow-up with a larger patient cohort is required to better establish the long-term benefit of the procedure.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Attenello FJ, McGirt MJ, Gathinji M, Datoo G, Atiba A, Weingart J. Outcome of chiari-associated syringomyelia after hindbrain decompression in children: Analysis of 49 consecutive cases. Neurosurgery. 2008. 62: 1307-13
2. Ball MJ, Dayan AD. Pathogenesis of syringomyelia. Lancet Lond Engl. 1972. 2: 799-801
3. Barbaro NM, Wilson CB, Gutin PH, Edwards MS. Surgical treatment of syringomyelia. Favorable results with syringoperitoneal shunting. J Neurosurg. 1984. 61: 531-8
4. Batzdorf U, Klekamp J, Johnson JP. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998. 89: 382-8
5. Chotai S, Chan EW, Ladner TR, Hale AT, Gannon SR, Shannon CN. Timing of syrinx reduction and stabilization after posterior fossa decompression for pediatric chiari malformation Type I. J Neurosurg Pediatr. 2020. 26: 193-9
6. Durham SR, Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of chiari malformation Type I in pediatric patients: A meta-analysis. J Neurosurg Pediatr. 2008. 2: 42-9
7. Ellenbogen RG, Armonda RA, Shaw DW, Winn HR. Toward a rational treatment of chiari I malformation and syringomyelia. Neurosurg Focus. 2000. 8: E6
8. Gil Z, Rao S, Constantini S. Expansion of chiari I-associated syringomyelia after posterior-fossa decompression. Childs Nerv Syst. 2000. 16: 555-8
9. Hankinson T, Tubbs RS, Wellons JC. Duraplasty or not? An evidence-based review of the pediatric chiari I malformation. Childs Nerv Syst. 2011. 27: 35-40
10. Heiss JD, Suffredini G, Smith R, DeVroom HL, Patronas NJ, Butman JA. Pathophysiology of persistent syringomyelia after decompressive craniocervical surgery. Clinical article. J Neurosurg Spine. 2010. 13: 729-42
11. Klekamp J, Flint G, Rusbridge C, editors. Hindbrain-related syringomyelia. Syringomyelia. A disorder of CSF circulation. Berlin: Springer; 2014. p. 141-66
12. Logue V, Edwards MR. Syringomyelia and its surgical treatment--an analysis of 75 patients. J Neurol Neurosurg Psychiatry. 1981. 44: 273-84
13. Matsumoto T, Symon L. Surgical management of syringomyelia--current results. Surg Neurol. 1989. 32: 258-65
14. Mazzola CA, Fried AH. Revision surgery for chiari malformation decompression. Neurosurg Focus. 2003. 15: E3
15. Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994. 80: 3-15
16. Schuster JM, Zhang F, Norvell DC, Hermsmeyer JT. Persistent/ recurrent syringomyelia after chiari decompression-natural history and management strategies: A systematic review. Evid Based Spine Care J. 2013. 4: 116-25
17. Sgouros S, Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995. 82: 1-10
18. Soleman J, Roth J, Bartoli A, Rosenthal D, Korn A, Constantini S. Syringo-subarachnoid shunt for the treatment of persistent syringomyelia following decompression for chiari Type I malformation: Surgical results. World Neurosurg. 2017. 108: 836-43
19. Soleman J, Roth J, Constantini S. Syringo-subarachnoid shunt: How I do it. Acta Neurochir (Wien). 2019. 161: 367-70
20. Thompson DN, Di Rocco C, Pang D, Rutka JT, editors. Chiari I malformation and associated syringomyelia. Textbook of pediatric neurosurgery. Berlin: Springer International Publishing; 2018. p. 1-32
21. Tubbs RS, McGirt MJ, Oakes WJ. Surgical experience in 130 pediatric patients with chiari I malformations. J Neurosurg. 2003. 99: 291-6
22. Wetjen NM, Heiss JD, Oldfield EH. Time course of syringomyelia resolution following decompression of chiari malformation Type I. J Neurosurg Pediatr. 2008. 1: 118-23
23. Williams B. Simultaneous cerebral and spinal fluid pressure recordings 2. Cerebrospinal dissociation with lesions at the foramen magnum. Acta Neurochir (Wien). 1981. 59: 123-42
24. Williams B. Syringomyelia. Neurosurg Clin N Am. 1990. 1: 653-85






Azeem
Posted October 2, 2023, 6:28 pm
Excellent job…is there any 5 yr follow up..