- Department of Neurosurgery, Salford Royal Foundation Trust, Manchester, United Kingdom
- Department of Neurosurgery, North Bristol NHS Trust, Bristol, United Kingdom.
Correspondence Address:
Siddharth Sinha
Department of Neurosurgery, Salford Royal Foundation Trust, Manchester, United Kingdom
DOI:10.25259/SNI_378_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Siddharth Sinha1, Venkat Iyer2, K. Joshi George1. Foramen magnum osteochondroma causing myelopathy in a patient with hereditary multiple exostoses. 18-Sep-2020;11:296
How to cite this URL: Siddharth Sinha1, Venkat Iyer2, K. Joshi George1. Foramen magnum osteochondroma causing myelopathy in a patient with hereditary multiple exostoses. 18-Sep-2020;11:296. Available from: https://surgicalneurologyint.com/surgicalint-articles/10266/
Abstract
Background: Osteochondromas are commonly occurring benign bone tumors which may be either a solitary lesion or occur due to association with hereditary multiple exostoses (HMEs). There have been several reported cases of spinal osteochondromas, but intracranial lesions are rare.
Case Description: A 51-year-old male with a history of multiple osteochondromas presented with myelopathy. He had an exostosis arising from the foramen magnum causing compression of the cervical spinal cord that was successfully removed. Genetic testing revealed that he had HMEs.
Conclusion: Osteochondromas of the skull are extremely rare. However, parts of the foramen magnum ossify in cartilage and can give rise to an osteochondroma. Here, we present a patient with HMEs who developed cervical myelopathy due to an osteochondroma arising from the foramen magnum. Due to the cartilaginous ossification of the foramen magnum, clinicians should be aware that osteochondromas can occur in this location and potentially give rise to cervical myelopathy.
Keywords: Exostoses, Osteochondroma, Myelopathy
INTRODUCTION
Osteochondromas are benign bone tumors occurring in 3% of the general population; they account for 30% of all benign bone tumors.[
CASE DESCRIPTION
A 51-year-old male presented with a 12-year history of difficulty climbing stairs, 12 months of clumsiness of both hands, and 9 months of shooting pain into the radial aspect of his left forearm, along with right-handed weakness/grip. He had historically had osteochondromas removed from his right iliac crest and left scapula, 12 and 11 years ago, respectively. He also gave a history of a bony growth on his forearm which had been previously removed.
Neurological examination
On examination, he was 5 foot 4 inches in height with bowing of both forearms and legs, and exaggerated curvature of the medial border of both feet. He exhibited a scissoring gait with spasticity (i.e., no motor deficit but hyperactivity of reflexes with bilateral Babinski responses) more right sided along with right medial thigh atrophy. The sensory examination was intact.
Diagnostic studies
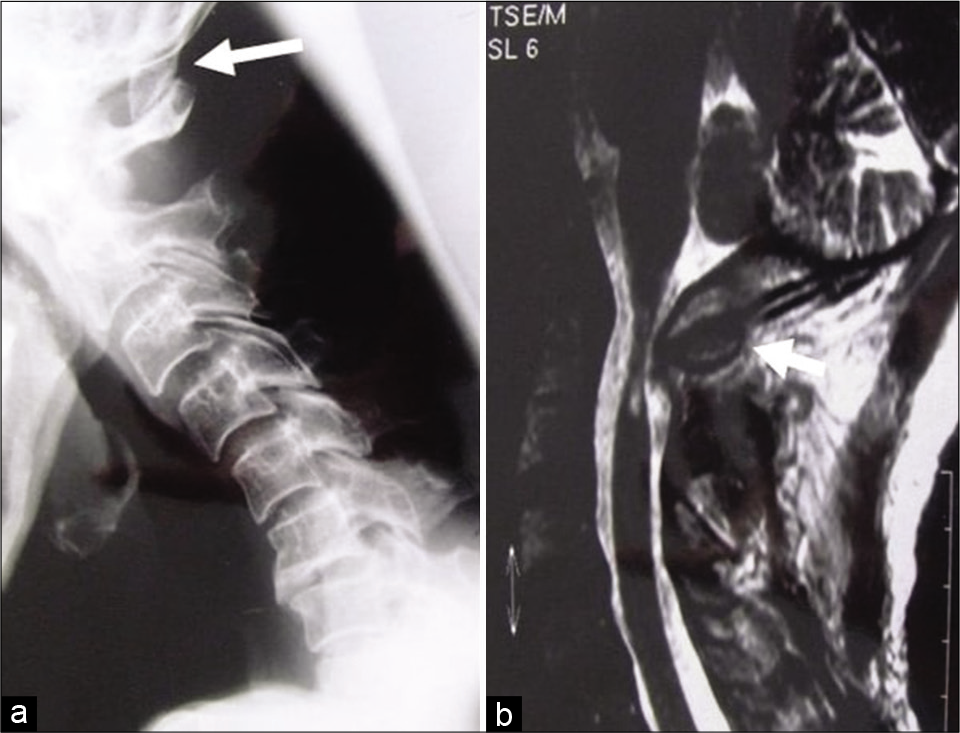
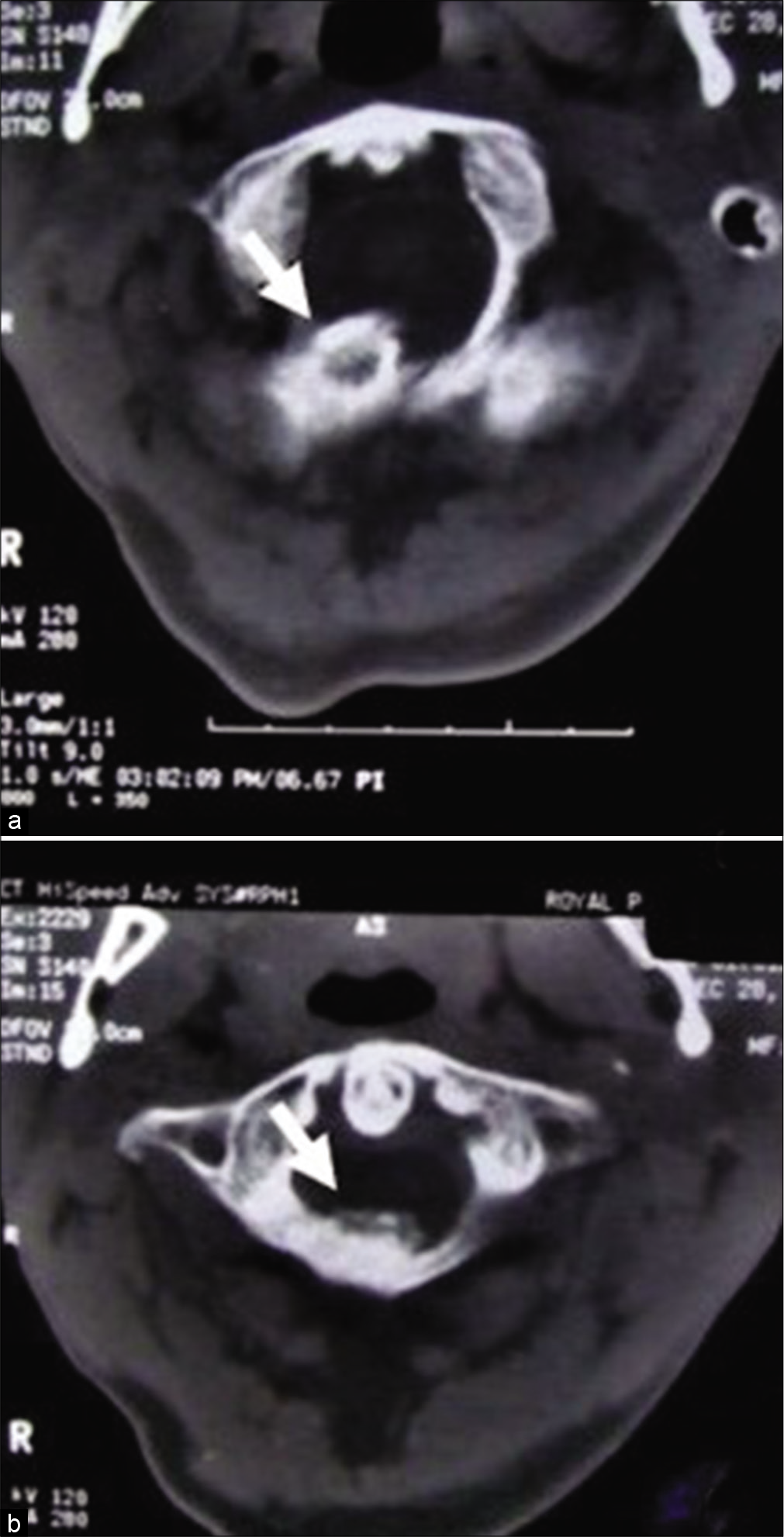
Plain X-ray of the cervical spine [
Figure 3:
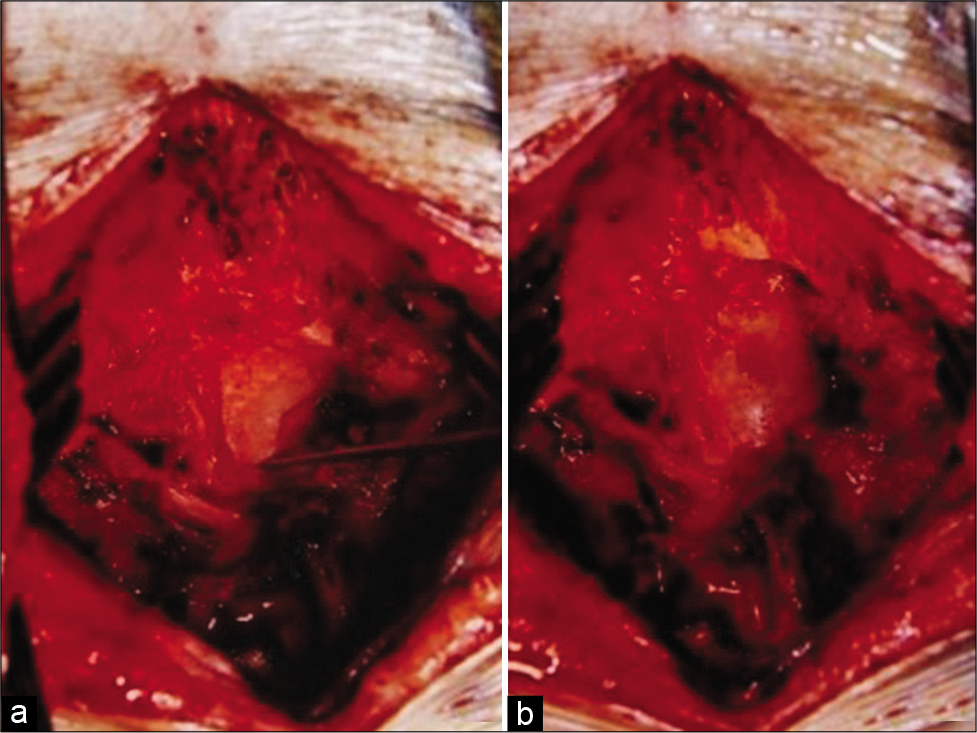
(a) Intraoperative photograph after removing the posterior arch of C1 lamina shows the pointer identifying the tip of the exostosis arising from the posterior lip of the foramen magnum. (b) Intraoperative photograph after removing the osteochondroma shows the decompressed cervical spinal cord.
Surgery
The patient underwent removal of the C1 lamina en bloc laminectomy; it revealed a bony exostosis covered with a cartilaginous cap arising from the posterior lip of the foramen magnum that was drilled away achieving good decompression of the dura surrounding the spinal cord [
Histopathology
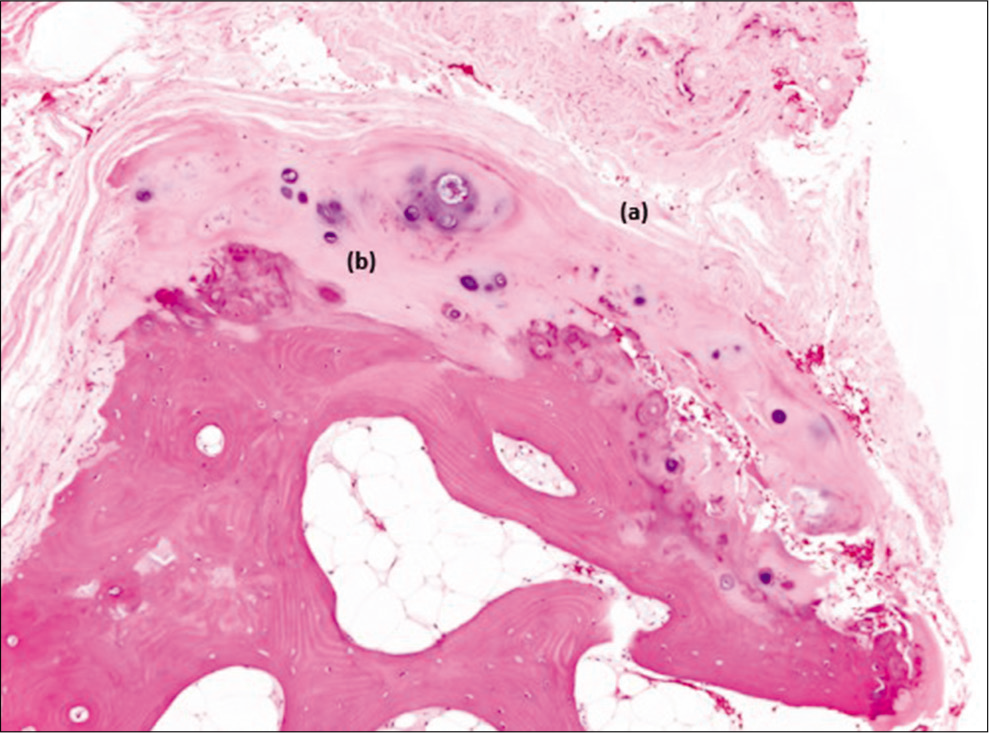
The histopathological report confirmed the diagnosis of osteochondroma [
DISCUSSION
Osteochondroma is the most common benign bone tumor. It is a cartilage-tipped exostosis and can be sessile or pedunculated with a cancellous structure that is well formed with a complete cortex. The stalk of an exostosis must be in direct continuity with the underlying cortex and medullary canal to be considered a true osteochondroma.[
Intracranial osteochondromas are very rare, accounting for 0.1–0.2% of all intracranial tumors.[
Hongo et al. (2015) and an additional seven cases (including our report) demonstrated that 43.3% of these lesion originating from the skull convexity, with the remainder originating from the falx cerebri (13.3%), parasellar (10%), posterior clinoid (10%), foramen magnum (6.7%), sella turcica (6.7%), middle fossa (3.3%), petrous bone (3.3%), and suprasellar (3.3%) regions.[
CONCLUSION
Here, we report a foramen magnum osteochondroma in a patient with HME. Spinal surgeons should be aware that osteochondromas can arise from this location precipitating the onset of cervical myelopathy.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amita R, Sandhyamani S, Easwer H, Nair S, Praveen A, Kapilamoorthy T. Giant intracranial osteochondroma: A case report with review of literature. Indian J Neurosurg. 2017. 3: 169-70
2. Beck DW, Dyste GN. Intracranial osteochondroma: MR and CT appearance. AJNR Am J Neuroradiol. 1989. 10: S7-8
3. Beltrami G, Ristori G, Scoccianti G, Tamburini A, Capanna R. Hereditary multiple exostoses: A review of clinical appearance and metabolic pattern. Clin Cases Miner Bone Metab. 2016. 13: 110-8
4. Hongo H, Oya S, Abe A, Matsui T. Solitary osteochondroma of the skull base: A case report and literature review. J Neurol Surg Rep. 2015. 76: e13-7
5. Hori YS, Ebisudani Y, Aoi M. Joint capsule-like intracranial osteochondroma mimicking cystic meningioma. World Neurosurg. 2017. 108: 985.e9-11
6. Kitsoulis P, Galani V, Stefanaki K, Paraskevas G, Karatzias G, Agnantis NJ. Osteochondromas: Review of the clinical, radiological and pathological features. In Vivo. 2008. 22: 633-46
7. Kumar S, Shah A, Patel A, Shah U. CT and MR images of the flat bone Osteochondromata from head to foot: A pictorial essay. Indian J Radiol Imaging. 2006. 16: 589
8. Lotfinia I, Vahedi A, Aeinfar K, Tubbs RS, Vahedi P. Cervical osteochondroma with neurological symptoms: Literature review and a case report. Spinal Cord Ser Cases. 2017. 3: 16038
9. Lotfinia I, Vahedi P, Tubbs RS, Gavame M, Vahedi A. Basioccipital bone osteochondroma growing into the foramen magnum. Surg Neurol Int. 2012. 3: 21
10. Murphey MD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: Variants and complications with radiologic-pathologic correlation. Radiographics. 2000. 20: 1407-34
11. Padhya TA, Athavale SM, Kathju S, Sarkar S, Mehta AR. Osteochondroma of the skull base. Otolaryngol Neck Surg. 2007. 137: 166-8
12. Sullivan JC, Goldsmith J, Rojas R, Varma H, Kasper EM. Intracranial dural parafalcine chondroma: Case report and systematic review of the literature. World Neurosurg. 2019. 122: 1-7
13. Venkata R, Garikaparthi S, Parvatala A, Kakarala S, Duttaluru S, Chinnam A. Giant intracranial osteochondroma: A case report and review of the literature. Surg Neurol Int. 2011. 2: 118
14. Zanotti MC, Melamed I, Diomin V, Walter E, Baraf L, Frenkel M. A multidisciplinary approach for the treatment of young patients with suprasellar osteochondroma. Childs Nerv Syst. 2018. 34: 559-63