- Department of Neurosurgery, Centro Hospitalar Lisboa Norte, Lisboa, Portugal
Correspondence Address:
Diogo Simão
Department of Neurosurgery, Centro Hospitalar Lisboa Norte, Lisboa, Portugal
DOI:10.4103/sni.sni_218_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Diogo Simão, Joaquim C. Teixeira, Alexandre R. Campos, Domingos Coiteiro, Maria M. Santos. Fourth ventricle neurocysticercosis: A case report. 03-Oct-2018;9:201
How to cite this URL: Diogo Simão, Joaquim C. Teixeira, Alexandre R. Campos, Domingos Coiteiro, Maria M. Santos. Fourth ventricle neurocysticercosis: A case report. 03-Oct-2018;9:201. Available from: http://surgicalneurologyint.com/surgicalint-articles/9029/
Abstract
Background:Neurocysticercosis (NCC) is the most common helminthic disease of the nervous system in humans and it is caused by the larvae of the pork tapeworm, Taenia solium. We present a case of microsurgical removal of a fourth ventricle NCC cyst combined with an endoscopic third ventriculostomy (ETV) to treat hydrocephalus.
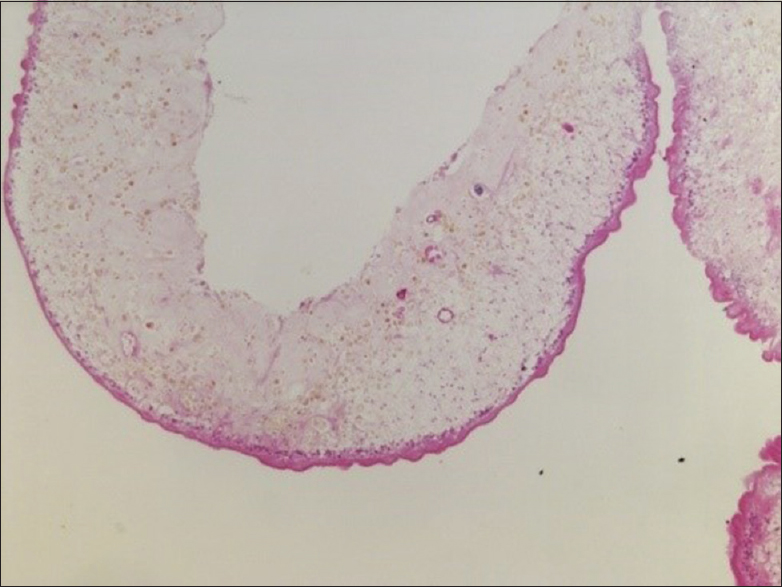
Case Description:A 36-year-old woman presented to the emergency room with headache and decreased visual acuity over the last 4 months. A brain magnetic resonance imaging showed obstructive hydrocephalus apparently correlated to a mobile, cystic lesion of the fourth ventricle. In the same operative time, an ETV and a suboccipital craniotomy were performed in order to remove the lesion and to treat the hydrocephalus. The cyst was completely removed and pathologically identified as a T. solium cyst. The early postoperative course was uneventful and she was discharged asymptomatic and off anthelmintic medication. Five weeks later, the patient returned with hydrocephalus recurrence and was successfully retreated with an ETV. At 5-month follow-up, she remains asymptomatic and has no evidence of persistent disease or hydrocephalus recurrence.
Conclusion:Intraventricular neurocysticercosis is, typically, a surgical disease. For cysts located on the fourth ventricle, a suboccipital craniotomy and a telovelar approach remains a valid option. Cyst removal does not necessarily resolve the hydrocephalus problem. ETV offers an option to the classic shunt placement approach and was shown to be effective even on hydrocephalus recurrence.
Keywords: Fourth ventricle, intraventricular neurocysticercosis, ventriculostomy
INTRODUCTION
Neurocysticercosis (NCC) is the most common helminthic disease of the nervous system in humans and is caused by the larvae of the pork tapeworm Taenia solium.[
CASE
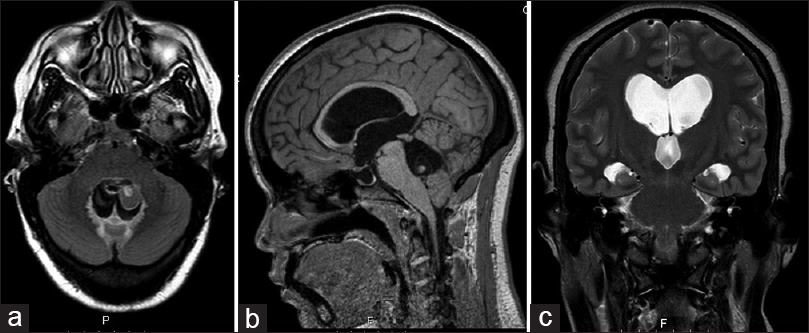
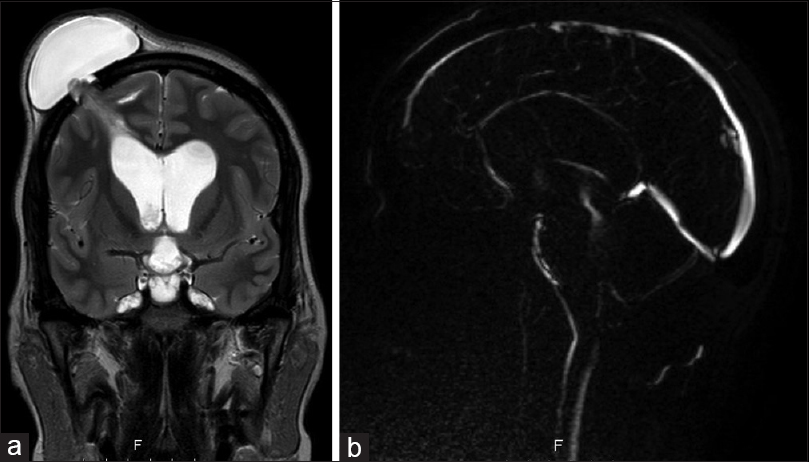
A 36-year-old female was referred to our emergency room by her homeland local hospital in Cape Verde (West Africa), with complaints of headaches and diminished vision over the last 4 months. On neurological examination, she had impaired visual acuity (right eye: 6/10; left eye: 7/10) and bilateral papilledema. No other neurological signs were observed. The brain magnetic resonance imaging (MRI) revealed hydrocephalus apparently caused by a well-defined cystic lesion in the fourth ventricle. The lesion was hypointense on T1-weighted and T2-weighted MRI sequences and had a peripheral millimetric solid component hyperintense on both sequences [
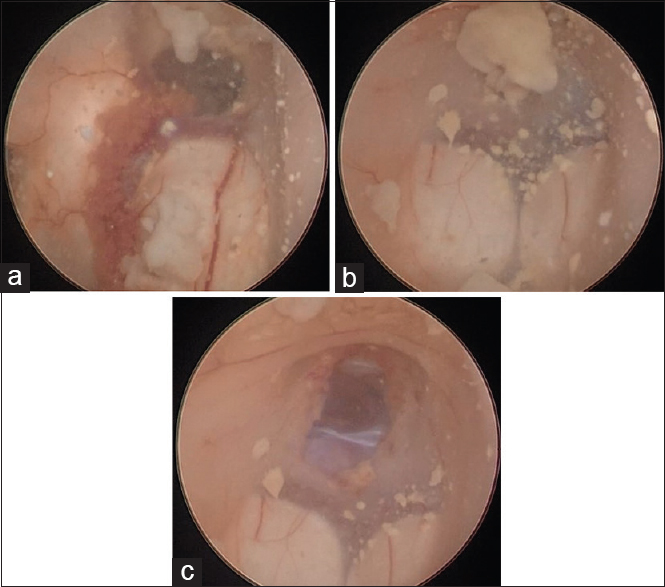
A surgical plan was elaborated to solve both problems – the hydrocephalus and the intraventricular cyst – at the same surgical time. Initially, an uneventful ETV was performed and intraventricular CSF was collected for further laboratorial analyses. No external drainage was left in place. The patient was then placed in prone position and underwent a suboccipital median craniotomy. Using microsurgical technique, a telovelar approach to the fourth ventricle was performed. The cyst microdissection was particularly challenging due to its thin wall and partial adherence to the surrounding ependyma, which contributed to an intraventricular cyst rupture by the end of its total removal. Extensive irrigation of the ventricle space with Ringer's solution was performed to avoid intraventricular dissemination.
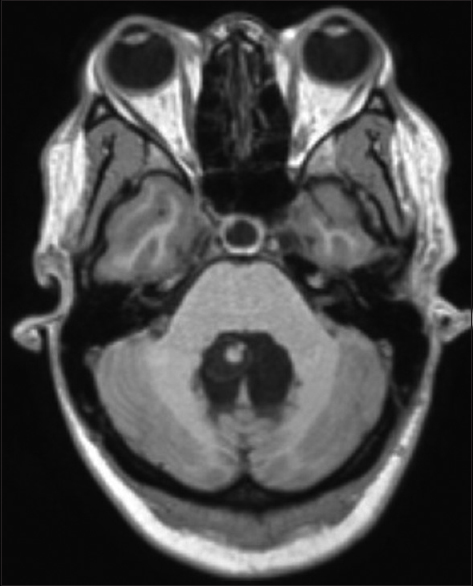
The patient had an uneventful postoperative course. The CSF analysis showed no alterations. Postop MRI showed a total removal of the cyst lesion and reduction of the ventricle dimensions [
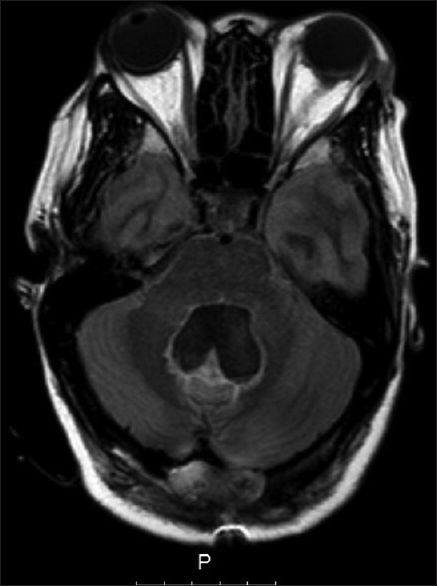
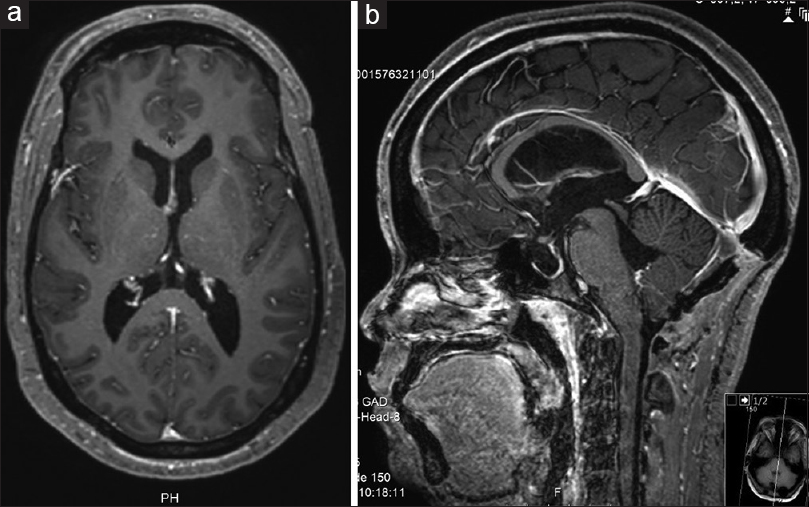
Five weeks after discharge, she presented back, complaining of headaches and worsening of vision again. A significant pseudomeningocele with no CSF leak was visible underneath the frontal surgical wound. A bilateral papilledema was evident in the ophthalmological examination. The MRI showed hydrocephalus recurrence with no evidence of CSF flow through the previous ventriculostomy stoma [
She has been followed up for 5 months now, remaining asymptomatic, with clearly defined optic discs on fundoscopy and has fully recovered visual acuity. The 5-month postop MRI ruled out hydrocephalus or any recurrent lesion [
DISCUSSION
NCC is the most common helminthic disease in the central nervous system and the most frequent preventable cause of epilepsy in the developing world.[
Depending on the affected compartment, NCC may be classified as intraparenchymal or extraparenchymal and the latter subdivided in intraventricular, subarachnoid and spinal forms. Extraparenchymal disease is known to be associated with more severe complications, such as hydrocephalus, arachnoiditis, and ventriculitis,[
Surgical treatment in extraparenchymal NCC is the standard of care to intraventricular cysts, hydrocephalus due to racemose cysts, or hydrocephalus due to ependymitis.[
Minimally invasive endoscopic removal of intraventricular cysts has emerged as the preferred surgical approach, especially if the cysts are in the lateral or third ventricles.[
Despite some referred negative effects of an intraoperative cyst rupture,[
Medical treatment for NCC remains controversial. In active intraparenchymal disease, anthelmintic medication has shown to prevent epilepsy and reduce the number of viable lesions.[
CONCLUSION
Treatment of intraventricular NCC involves cysts removal and treatment of associated hydrocephalus. In the presence of a removable single intraventricular cyst, anthelminthic medication is normally not necessary. Endoscopic techniques to treat hydrocephalus, such as ETV, should be preferred to shunt placement. We present a case in which combined microscopic and endoscopic approaches were performed for removal of a fourth ventricle cysticercus and treatment of the associated hydrocephalus. Our case is an example that failure at a first ETV does not make a second attempt futile and that cyst rupture does not necessarily lead to a worse outcome, although its role in hydrocephalus relapse is uncertain.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Last accessed on 2018 Aug 01. Available from: https://www.aan.com/Guidelines/home/GetGuidelineContent/592.
2. Apuzzo ML, Dobkin WR, Zee CS, Chan JC, Gianotta SL, Weiss MH. Surgical considerations in treatment of intraventricular cysticercosis. An analysis of 45 cases. J Neurosurg. 1984. 60: 400-7
3. Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: Treatment of parenchymal neurocysticercosis. Neurology. 2013. 80: 1424-9
4. Bergsneider M. Endoscopic removal of cysticercal cysts within the fourth ventricle. Technical note. J Neurosurg. 1999. 91: 340-5
5. Citow JS, Johnson JP, McBride DQ, Ammirati M. Imaging features and surgery-related outcomes in intraventricular neurocysticercosis. Neurosurg Focus. 2002. 12: 1-8
6. Coyle CM. Neurocysticercosis: An update. Curr Infect Dis Rep. 2014. 16: 437-
7. De Araujo AL, Rodrigues RS, Marchiori E, Pinheiro RA, Flores M, Alves JR. Migrating intraventricular cysticercosis. Arq Neuropsiquiatr. 2008. 66: 111-3
8. DeGiorgio CM, Houston I, Oviedo S, Sorvillo F. Deaths associated with cysticercosis. Report of three cases and review of the literature. Neurosurg Focus. 2002. 12: e2-
9. Fleury A, Escobar A, Fragoso G, Sciutto E, Larralde C. Clinical heterogeneity of human neurocysticercosis results from complex interactions among parasite, host and environmental factors. Trans R Soc Trop Med Hyg. 2010. 104: 243-50
10. Franko LR, Pandian B, Gupta A, Savastano LE, Chen KS, Riddell J. Posterior fossa craniotomy for adherent fourth ventricle neurocysticercosis. Oper Neurosurg. 2018. p.
11. Garcia HH, Evans CA, Nash TE, Takayanagui OM, White AC, Botero D. Current consensus guidelines for the treatment of neurocysticercosis. Rev Med Microbiol. 2002. 15: 747-56
12. Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004. 350: 249-58
13. Goel RK, Ahmad FU, Vellimana AK, Suri A, Chandra PS, Kumar R. Endoscopic management of intraventricular neurocysticercosis. J Clin Neurosci. 2008. 15: 1096-101
14. Gripper LB, Welburn SC. Neurocysticercosis infection and disease – A review. Acta Tropica. 2017. 166: 218-24
15. Hanak BW, Walcott BP, Codd PJ, Jones PS, Nahed BV, Butler WE. Fourth ventricular neurocysticercosis presenting with acute hydrocephalus. J Clin Neurosci. 2011. 18: 867-9
16. Jensen TO, Post JJ. Intraventricular neurocysticercosis: Presentation, diagnosis and management. Asian Pac J Trop Med. 2016. 9: 815-8
17. Kaif M, Husain M, Ojha BK. Endoscopic management of intraventricular neurocysticercosis. Turk Neurosurg. 2018. p.
18. Loyo M, Kleriga E, Estañol B. Fourth ventricular cysticercosis. Neurosurgery. 1980. 7: 456-8
19. Madrazo I, Gracia JA, Sondoval M, Lopez Vega FJ. Intraventricular cysticercosis. Neurosurgery. 1983. 12: 148-52
20. Nash T, Ware JM, Mahanty S. Intraventricular neurocysticercosis: Experience and long-term outcome from a tertiary referral center in the United States. Am J Trop Med Hyg. 2018. 98: 1755-62
21. Psarros TG, Zouros A, Coimbra C. Neurocysticercosis. A neurosurgical perspective. South Med J. 2003. 96: 1019-22
22. Rajshekhar V. Surgical management of neurocysticercosis. Int J Surg. 2010. 8: 100-4
23. Rana S, Prasad A, Brar R, Rathore D, Dwivedi A. Caught in the act: Migrating intraventricular neurocysticercosis causing intermittent unilateral hydrocephalus due to foramen of Monro obstruction. Acta Neurol Belg. 2018. 118: 509-11
24. Rizvi SA, Saleh AM, Frimpong H, Al Mohiy HM, Ahmed J, Edwards RD. Neurocysticercosis: A case report and brief review. Asian Pac J Trop Med. 2016. 9: 100-2
25. Thomas B, Krishnamoorthy T. Migrating intraventricular cysticercus during MRI. Neurology. 2005. 65: 1321-
26. White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2018. 98: 945-66
27. Last accessed on 2018 May 11. Available from: http://www.who.int/features/factfiles/neurocysticercosis/en/.
28. Xiao A, Xiao J, Zhang X, You C. The surgical value of neurocysticercosis: Analyzing 10 patients in 5 years. Turk Neurosurg. 2016. 26: 744-9