- Radio Neurosurgery Unit, National Institute of Neurology and Neurosurgery “Dr. Manuel Velasco Suarez”, Mexico City, Mexico.
DOI:10.25259/SNI_247_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Guillermo Axayacalt Gutierrez-Aceves, Alejandro Rodriguez-Camacho, Miguel Angel Celis-Lopez, Sergio Moreno-Jimenez, Jose Alfredo Herrera-Gonzalez. Frameless radiosurgical third ventriculostomy: Technical report. 18-Nov-2020;11:398
How to cite this URL: Guillermo Axayacalt Gutierrez-Aceves, Alejandro Rodriguez-Camacho, Miguel Angel Celis-Lopez, Sergio Moreno-Jimenez, Jose Alfredo Herrera-Gonzalez. Frameless radiosurgical third ventriculostomy: Technical report. 18-Nov-2020;11:398. Available from: https://surgicalneurologyint.com/surgicalint-articles/10401/
Abstract
Background: We describe the technical report and results of the first image-guided, linear accelerator, frameless radiosurgical third ventriculostomy.
Methods: We report a 20 years old man, with diplopia, balance disturbances, and limitation for gaze supraversion. Magnetic resonance imaging resonance imaging of the brain and cranial computed tomography showed showed a left thalamic-midbrain lesion that caused partial compression of the Silvio aqueduct and mild ventricular dilatation. The biopsy revealed the diagnosis of pleomorphic xanthoastrocytoma. Before radical treatment of the tumor with fractionated stereotactic radiotherapy, the patient underwent to frameless radiosurgical third ventriculostomy, on the TrueBeam STX® platform with the ExacTrac localization system. The target used was the one defined on the floor of the third ventricle, at the midpoint between the mammillary bodies and the infundibular recess. The prescription dose was 120 Gy, given using a monoisocentric technique of multiple noncoplanar circular arches. The geometric arrangement of the plan consisted of 15 arches, with a 4 mm cone, distributed over a 110° table.
Results: There was symptomatic and image improvement two days after radiosurgery. On CT, a reduction in ventricular dilation was observed with a reduction in the Evans index from 0.39 (initial CT) to 0.29 (CT at 15 days). In 3.0T magnetic resonance image at 3 months, we showed the third ventriculostomy. There have been no treatment failures or complications.
Conclusion: It is possible to effectively perform the frameless radiosurgical third ventriculostomy without associated morbidity in the short term.
Keywords: Frameless, Linear accelerator, Obstructive hydrocephalus, Radiosurgery, Third ventriculostomy
INTRODUCTION
Conceived as a noninvasive treatment technique, with a greater analogy to surgery than radiotherapy, stereotactic radiosurgery (SRS) uses high doses of ionizing radiation, usually in a single fraction, directed at a stereotactically defined intracranial target. The gamma unit (Gamma Knife®), since its creation in the 60s, has been the most frequently used platform to do it, however, since its introduction in the 80s, the dedicated linear accelerator (LINAC-D), currently with technical and mechanical precision comparable to that of the gamma unit, has increased its use as a valuable tool in the practice of Radiosurgery (RS).[
The precise location of the target, the great spatial accuracy of the radiation beam, and the abrupt drop in the dose outside the volume of interest are essential characteristics of this procedure. To comply with these requirements, the use of a rigidly placed stereotactic guide device (stereotactic frame), other immobilization technology (mask), and/or an image-guided location system is essential.[
Since the 2000s, treatment localization systems (TLSs) for image-guided RS (IGRS) with mask-based immobilization (frameless) have been playing a very important role, as they achieve a degree of mechanical precision comparable to the stereotactic guide system with frame, while allowing noninvasive immobilization of the patient. The mechanical precision of these systems has been extensively studied.[
Radiosurgery finds its main indications in benign, malignant, and functional intracranial pathology since it has the capacity to eradicate or inactivate a tumor, to favor the obliteration of the anomalous vasculature in a malformative nest, to produce a precise lesion that allows interrupting neuronal pathways, or to generate a continuity solution on a specific target; this last characteristic has allowed us to transcend its indications and extend its applications.[
On this occasion, we report the procedure and results of the first image-guided (frameless) radiosurgical third ventriculostomy (IGRS-ThirdV), with LINAC TrueBEAM STx platform (Varian Medical Systems, Palo Alto, California, United States), and performed at the Institute National Neurology and Neurosurgery “Manuel Velasco Suarez” from Mexico City.
Description of the case
We present a 20-year-old man with a 1-month history of diplopia, balance disturbances, spatial disorientation, and limitation for conjugate gaze supraversion. Magnetic resonance imaging (MRI) of the brain and cranial computed tomography (CT) showed a left thalamic midbrain injury that caused partial compression of the Silvio aqueduct and mild ventricular dilation, with an Evans index (EI) of 0.39. The biopsy was obtained by neuronavigation and the histopathological report revealed the diagnosis of pleomorphic xanthoastrocytoma (WHO Grade II). Due to the aforementioned clinical and imaging characteristics and the patient’s functional status, 70% on the Karnofsky scale (KPS), we proposed performing a ETV, before radical treatment of the tumor with fractionated SRS (FSRT).
The patient declined the endoscopic procedure, so the radiosurgical procedure (IGRS-ThirdV) was chosen.
Pretreatment image, simulation tomography, and image fusion
Pretreatment brain MRI was performed with a 3-tesla (3.0 T) magnet resonator (General Electric [GE] Signa Twin Excite MRI Scanner, GE Medical Systems, Milwaukee, WI), with weighted volumetric sequence acquisition (SPGR) in simple T1, T2, and T1 with contrast, cuts with a thickness of 1 mm, matrix size of 512 × 512, pixel size of 0.45 mm, and without gap.
For noninvasive immobilization of the skull, a three-component thermoplastic mask for RS was formed (BrainLAB, Heimstetten, Germany), after placing the neck support, selected based on the patient’s anatomy. Subsequently, the method of immobilization was fixed to the mask base located on the universal table for tomography table from the same manufacturer. After the fixation system was placed, the images of the skull simulation CT (Siemens SOMATON Sensation 64 CT, Siemens Medical Solutions, Malvern, PA) were acquired with cuts of 0.7 mm thickness, reconstruction size of 512 × 512 matrix, and no spaces.
Coregistration of pretreatment MRI with simulation CT was performed using Brainlab Elements Image Fusion (version 3.0.1.6), which uses rigid registration methods to match anatomical structures present in different image sets, to obtain precise anatomical and geometric information for the proper definition of the target.
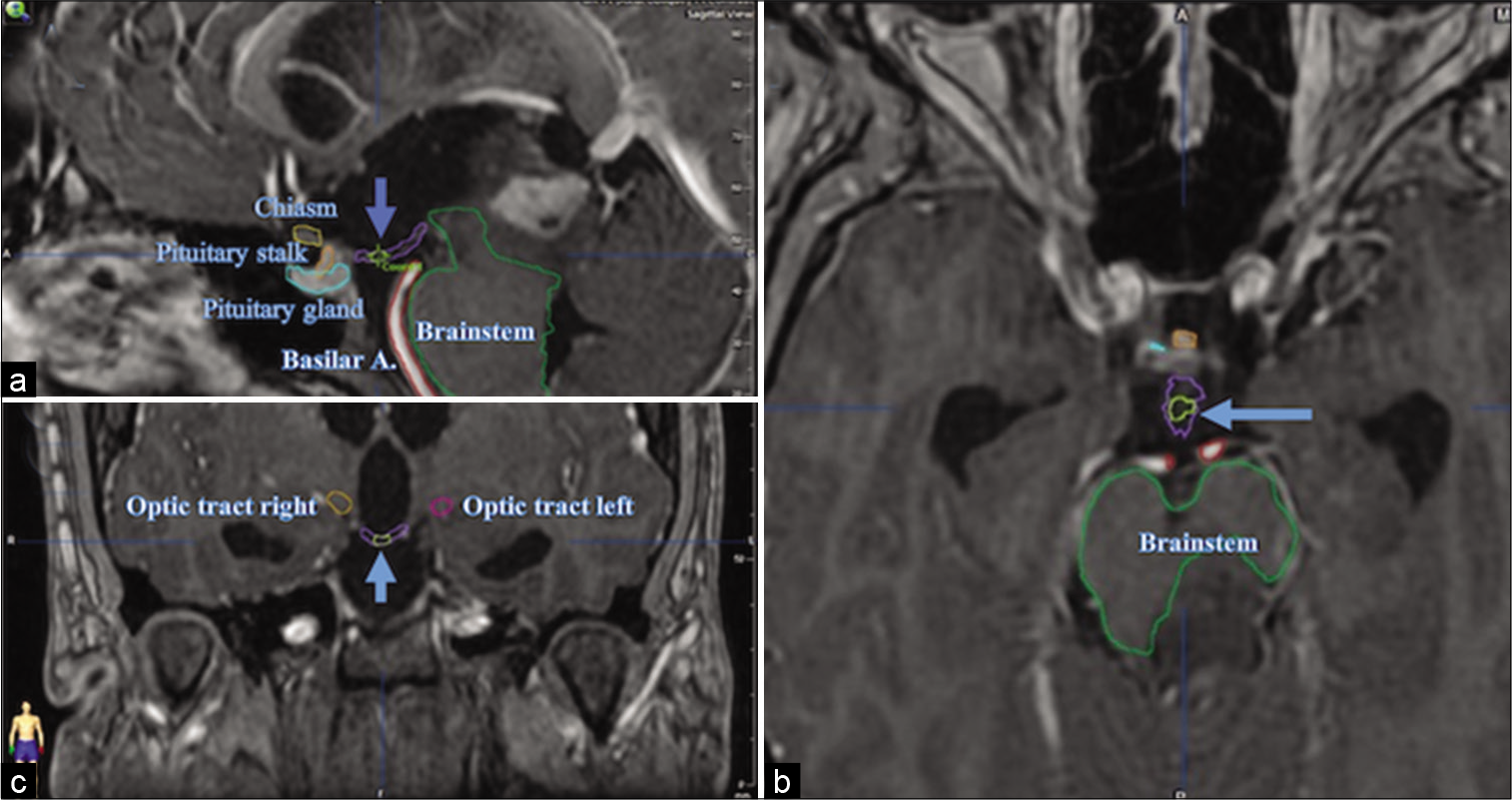
Definition of target volume and organs at risk
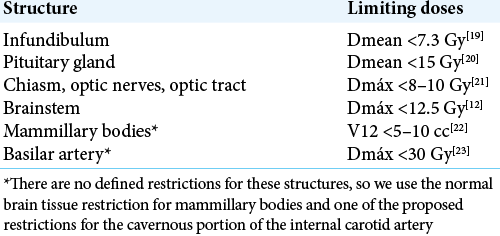
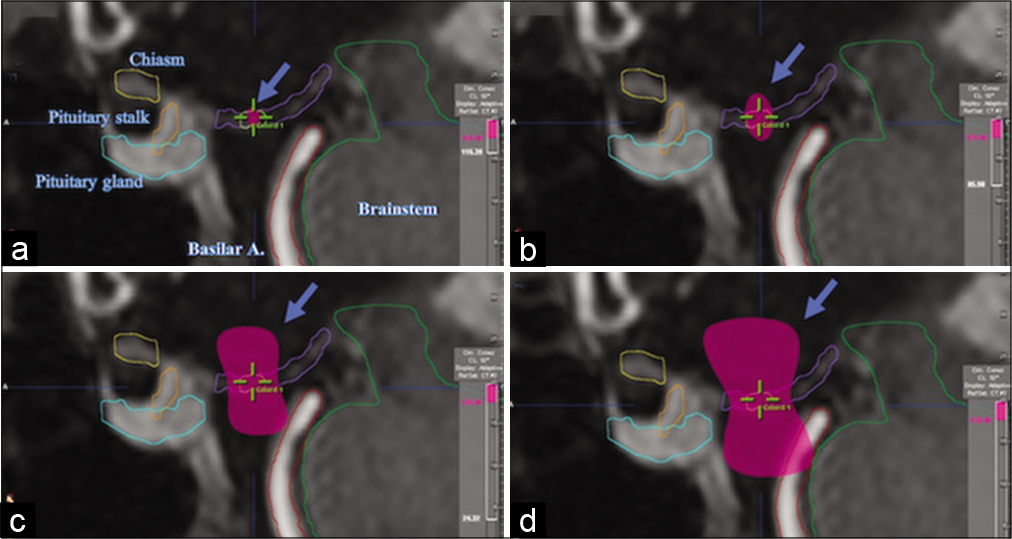
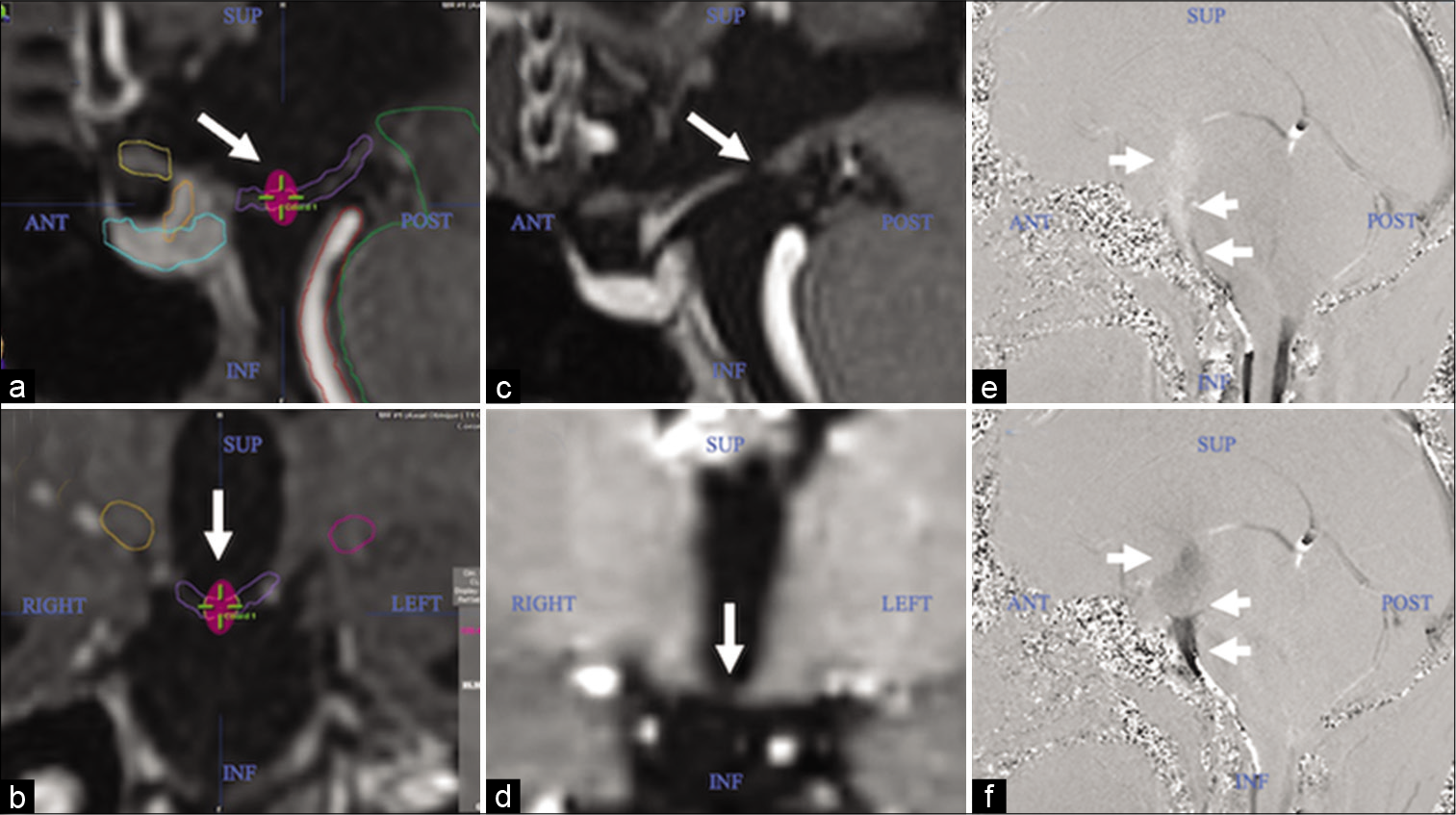
The definition of the target and the contouring of risk organs were performed with the BrainLAB iPlan RT Image delineation software (version 4.1.2.5). The target, as described in our previous article, was located on the brain MRI, in the SPGR sequence, weighted in T1 with contrast, in the mid-sagittal plane, and was defined on the floor of the third ventricle, at the point medium between the mammillary bodies and the infundibular recess [
Prescription dose, dose distribution, and treatment planning
The prescription dose to the isocenter was 120 Gy, given using a monoisocentric technique of multiple noncoplanar circular arches. The BrainLAB iPlan RT Dose system (Version 4.5.5) was used for the planning of treatment with cone collimation, which uses the Clarkson dose algorithm for the calculation and projection of the 3D distribution of the isodose curves.
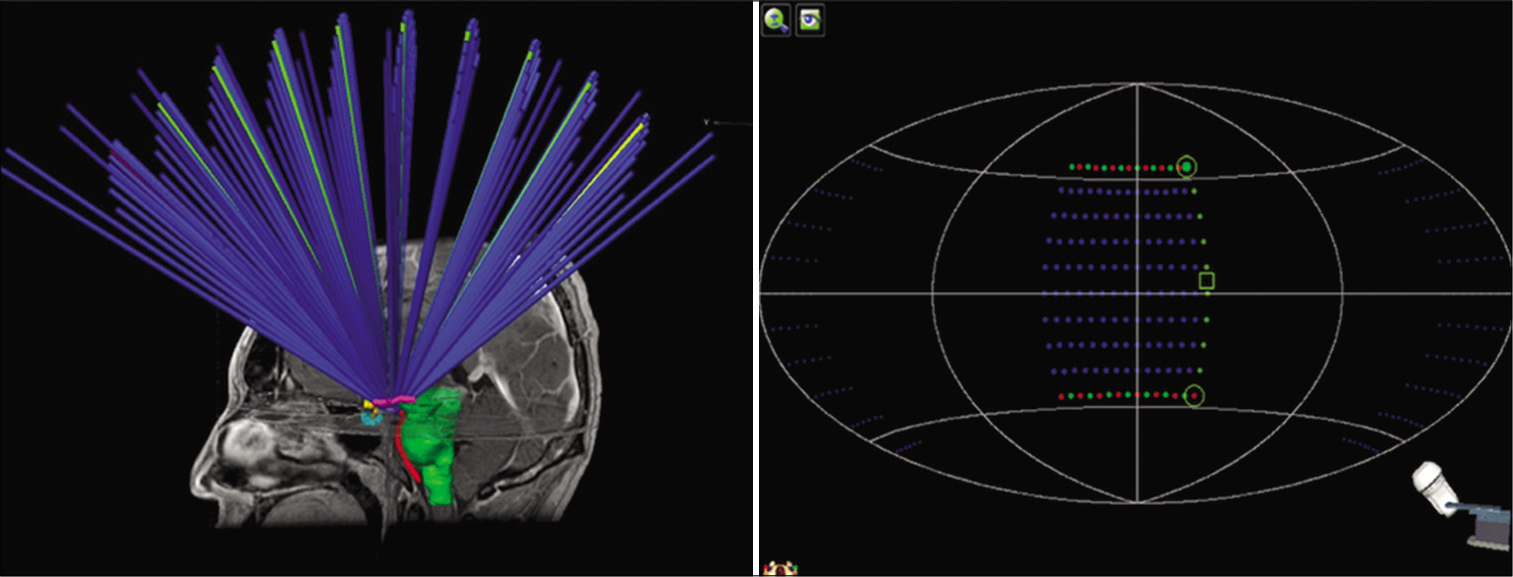
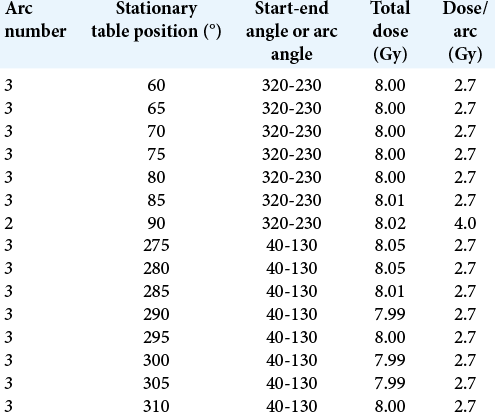
The geometric arrangement of the plan consisted of 15 arches, with the 4 mm cone, distributed at 110° of table, with table angles between 60° and 310°, each arc separated by 5° of table rotation and one gantry rotation 90°. The graphic representation of the arches arrangement is shown in [
Figure 2:
Graphic representation of the geometric arrangement of the noncoplanar circular arches for the treatment plan. Figure 3: Sagittal magnetic resonance imaging sections of the brain, contrasting T1, showing the 3D distribution of the 120 Gy (a), 85 Gy (b), 24 Gy (c), and 12 Gy isodosis curves (pink) (d).
The dose distribution was elongated on the Z-axis (craniocaudal), therefore, the 115 Gy curve crossed the entire floor of the third ventricle on the same axis (approximately 2 mm in length). The 85 Gy curve, even more elongated in the craniocaudal direction, and acquiring an “ellipsoidal” shape, managed to cover, above and below, the floor of the third ventricle, with the equivalent of its same thickness, with the aim of guarantee the coverage of spatial uncertainties associated with dosimetric parameters and natural movements during the respiratory cycle. A spatial “hourglass” distribution was generated in the 24 and 12 Gy isodose curves [
Treatment planning was carried out the same day the images were acquired, and 1 day before performing the IGRS-ThirdV procedure. The total planning time was approximately 180–240 min.
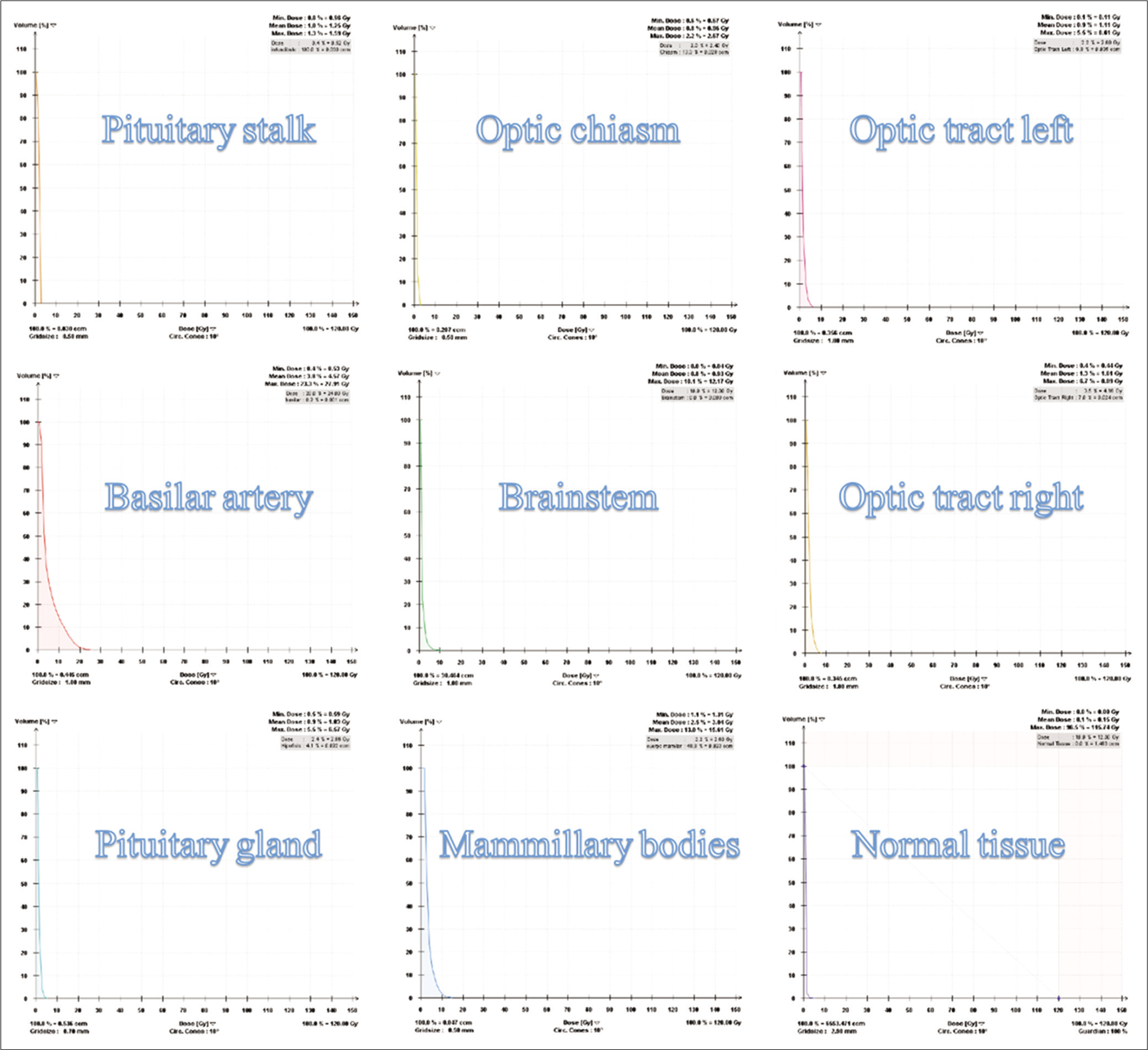
Dose-volume histograms (DVHs) of the radiosurgical third ventriculostomy
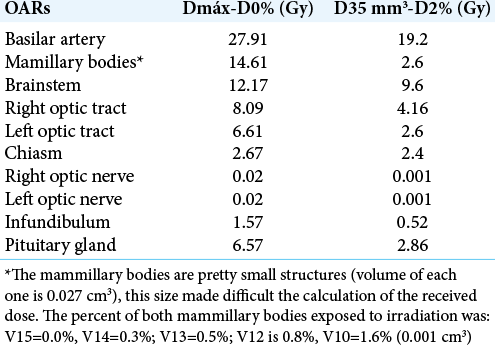
The evaluation of DVHs was carried out following the recommendations of report 91 of the International Commission on Radiation and Measurement Units (ICRU) for the prescription, recording and reporting of stereotactic treatments with small photon beams.[
Patient placement, treatment, and quality control
The patient positioned himself on the treatment table, placed the neck support, and was immobilized with the mask. Subsequently, the BrainLAB positioning arrangement for RS frameless was placed, which provides precise positioning information to the TLS.
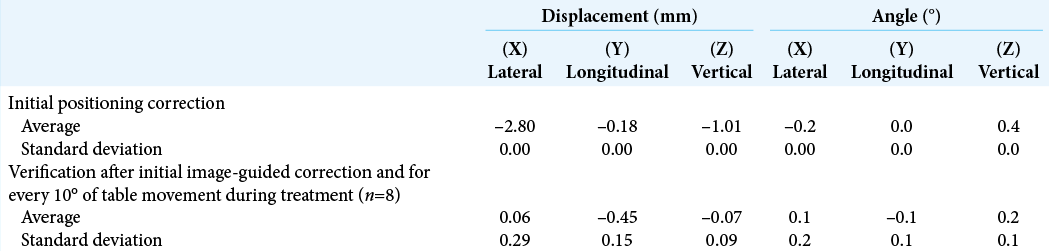
The TLS used for image guidance was the ExacTrac6.2 (ET) system (BrainLAB AG), which integrates an optical positioning system based on infrared (IR), a radiographic positioning system consisting of the acquisition of two oblique images kV X-ray stereoscopic (X-ray 6D) and a robotic stretcher that can move in six directions. The verification of the initial position and for each table movement was performed with the coregistration of the images acquired by the ET with the digitally reconstructed radiographs (DRRs) of the simulation CT, using 6° of-freedom fusion algorithms (6DOF).[
The IGRS-ThirdV was performed with a LINAC TrueBEAM STx (Varian Medical Systems, Palo Alto, California, United States), with 6X-FFF energy (without flattening filter), with 31,100 total MUs and a dose rate of 1200 UM/min, for a total beam time of 25.91 min, and a total treatment time of 78.56 min. During this time, there was communication with the patient to ensure that he was in a comfortable position and thus prevent any movement.
Before treatment, the quality control tests (QA) of the isocenter were carried out, based on: the calibration of the TLS isocenter, using the ET QA program; the Winston-Lutz (W-L) test, demonstrating a rotational precision of the gantry and the LINAC table <0.4 mm; and the “Detection of the W-L phantom” test, meeting the tolerance of 1 mm, between the ET (KV) isocenter and the LINAC (MV) isocenter, in three dimensions (3D) (X, Y, and Z).
Dosimetric studies showed a variation of the prescribed dose of less than 2%.
One day after the radiosurgical procedure, the left diencephalic lesion (pleomorphic xanthoastrocytoma) with a planning target volume of 12.3 ml, was treated with FSRT, using a volumetric intensity modulated arcotherapy technique, which consisted of two coplanar arches, with gantry rotation between 35° and 179°, collimator between 30° and 330°, and table at 0°. The prescription dose was 50.4 Gy, given in 28 fractions. The radiosurgical procedure was considered for the evaluation of the DVH of the fractional treatment, obtaining that in the sum plan, the final cumulative doses were below the tolerance doses for each OAR.
RESULTS
Postprocedure course
Clinical evaluation was performed every day during the first week, each week during the first month, then every 15 days for the 2nd month, and then 1 day after acquisition of neuroimaging studies. The follow-up by image consisted of acquiring a cranial CT at 2, 7, 15, and 30 days posttreatment, continuing the follow-up with the acquisition of MRI at 3, 6, 9, 12, 18, and 24 months.
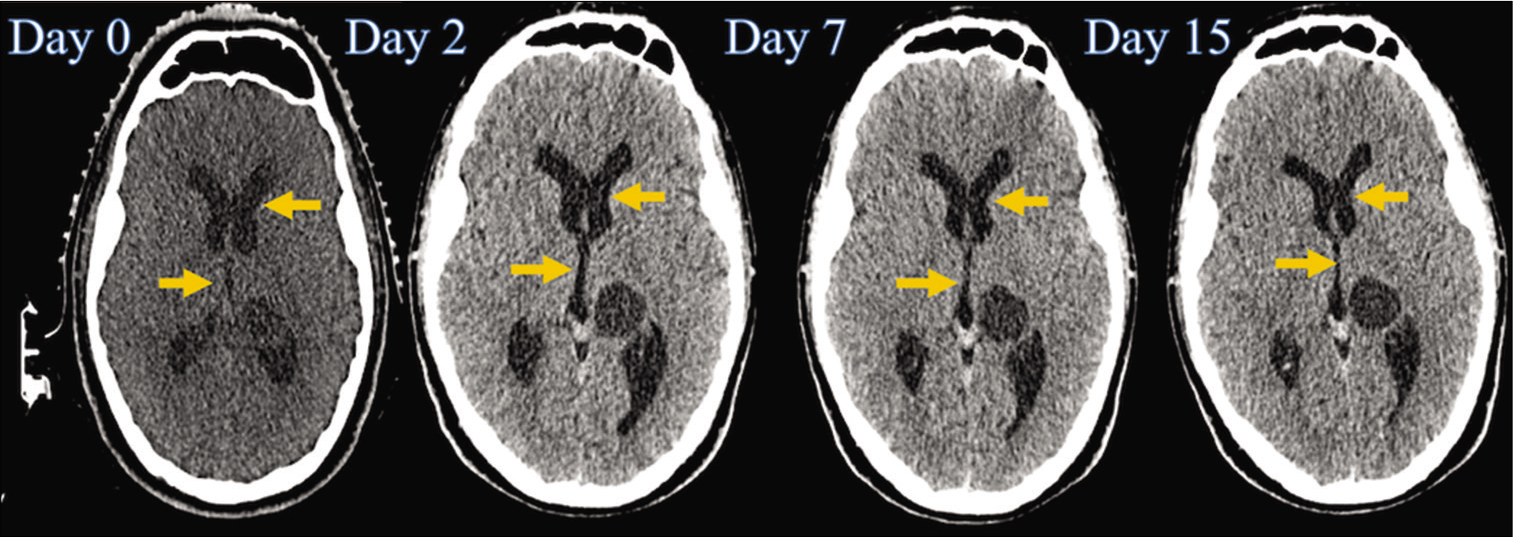
After the radiosurgical procedure, prednisone was started at a rate of 1 mg/kg/day with a dose reduction scheme (10 mg/ week). In the first clinical evaluation, the patient showed improvement in diplopia and balance disturbances, so the KPS scale score was increased (80%), and in the evaluation at 3 months, he was already free of symptoms ( 100% KPS). On skull CT, 2 days after treatment, we found a slight reduction in ventricular dilation with EI of 0.35; at 7 days, we found an even greater reduction in ventricular dilution with an EI of 0.32, and at 15 days, we showed the resolution of hydrocephalus, EI of 0.29, which has been maintained throughout the patient’s follow-up [
Figure 5:
Time line of the cranial tomography (before and after the procedure), where a progressive reduction in the size of the third ventricle and of the frontal horns of the lateral ventricles is observed (indicated with yellow arrows). However, de tumoral size does not have any changes (at this time, the patient was in treatment with conventional fractionation of the tumor).
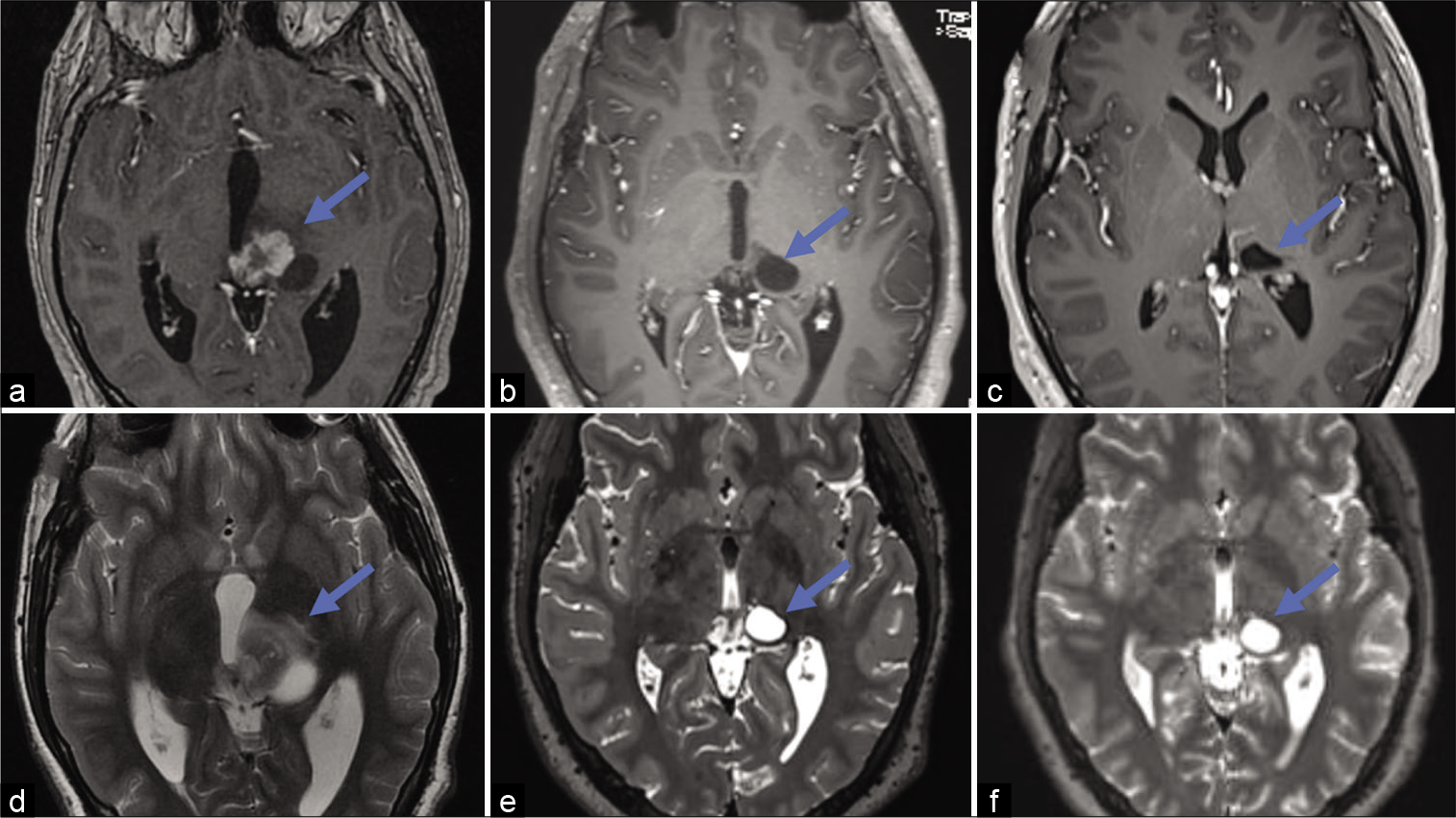
Regarding the evolution of pleomorphic xanthoastrocytoma, on MRI of the brain 3 months after treatment, we showed a partial response characterized by a reduction >50% of the lesion in its perpendicular diameters, with persistence only of the cystic portion of the disease, with a volume of 2.34 cm3, which has remained stable for 2 years of follow-up [
During the entire follow-up, the patient did not present clinical data of neurological progression or alterations secondary to RS. The patient was irradiated 2 years ago. Nowadays, he is a laws student in the Universidad Nacional Autonoma de Mexico (UNAM). He is an excellent student, with high performance, his recent score is 98 (98/100).
DISCUSSION
Endoscopic third ventriculostomy in obstructive hydrocephalus secondary to midline tumor pathology
Endoscopic third ventriculostomy combined with tumor biopsy, or resection, when feasible, is the recommended primary treatment in patients with tumors of the posterior region of the third ventricle and of posterior fossa in the midline that condition obstructive hydrocephalus.[
Radiosurgical third ventriculostomy with frame and stereotactic guide, and radiosurgical third ventriculostomy with image guided (frameless)
In general terms, the main technical differences between the SRS and the IGRS are the immobilization method (frame or mask) and the procedure guide (stereotactic or image guide). Placing an invasive stereotactic framework through mechanical fixation is safe and offers little morbidity, however, it does result in certain limitations regarding patient comfort and workflow. The introduction of frameless IGRS allows for noninvasive treatment, which does not require anesthesia and avoids the need for close patient monitoring.[
In 2012, we reported the results and procedure of the first SRSThirdV in a patient with pontine metastasis from clear cell renal cancer, causing compression of the fourth ventricle and partial obliteration of the Silvio aqueduct, with ventricular size index (VSI). On that occasion, 100 Gy were prescribed to the previously defined target, obtaining as a result subjective symptomatic improvement in the 1st week after the procedure, with elevation of the KPS (90%), as well as reduction of the VSI of 4% in the cranial CT at 7 days. The stoma, 2.63 mm, was evidenced in the 3-month MRI of control, and in the cine phase, the patent of the CSF circulation from the third ventricle to the interpeduncular cistern was observed. The VSI was maintained between 30 and 32% throughout its follow-up (8 months), and no complications were reported.[
CONCLUSION
The IGRS-ThirdV is a noninvasive and highly accurate technique, which in this case proved to be safe and effective, and whose main indication could be found in selected patients with mild obstructive hydrocephalus secondary to tumoral pathology.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barnett GH, Linskey ME, Adler JR, Cozzens JW, Friedman WA, Heilbrun MP. Stereotactic radiosurgery-an organized neurosurgery-sanctioned definition. J Neurosurg. 2007. 106: 1-5
2. Chen JC, Rahimian J, Rahimian R, Arellano A, Miller MJ, Girvigian MR. Frameless image-guided radiosurgery for initial treatment of typical trigeminal neuralgia. World Neurosurg. 2010. 74: 538-43
3. Deinsberger R, Tidstrand J. Linac radiosurgery as a tool in neurosurgery. Neurosurg Rev. 2005. 28: 79-88
4. El-Ghandour NM. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the treatment of obstructive hydrocephalus due to posterior fossa tumors in children. Childs Nerv Syst. 2011. 27: 117-26
5. Friehs GM, Noren G, Ohye C, Duma CM, Marks R, Plombon J. Lesion size following Gamma Knife treatment for functional disorders. Stereotact Funct Neurosurg. 1996. 66: 320-8
6. Frighetto L, de Salles A, Wallace R, Ford J, Selch M, CabatanAwang C. Linear accelerator thalamotomy. Surg Neurol. 2004. 62: 106-13
7. Gevaert T, Verellen D, Engels B, Depuydt T, Heuninckx K, Tournel K. Clinical evaluation of a robotic 6-degree of freedom treatment couch for frameless radiosurgery. Int J Radiat Oncol Biol Phys. 2012. 83: 467-74
8. Gilbo P, Zhang I, Knisely J. Stereotactic radiosurgery of the brain: A review of common indications. Chin Clin Oncol. 2017. 6: S14
9. Grunert P, Charalampaki P, Hopf N, Filippi R. The role of third ventriculostomy in the management of obstructive hydrocephalus. Minim Invasive Neurosurg. 2003. 46: 16-21
10. Gutierrez-Aceves GA, Moreno-Jimenez S, Celis MA, Hernandez-Bojorquez M. Radiosurgical third ventriculostomy: Technical note. Surg Neurol Int. 2012. 3: 121
11. Hacker F, Rosca F, Friesen S, Zygmanski P, Ramakrishna N. Accuracy assessment of a non-invasive image guided system for intra-cranial linac based stereotactic radiosurgery. Med Phys. 2006. 33: 2066
12. . International Commission on Radiation Units and Measurements. Report 91: Prescribing, recording, and reporting of stereotactic treatments with small photon beams. J ICRU. 2017. 14: 1-160
13. Isaacs AM, Bezchlibnyk YB, Yong H, Koshy D, Urbaneja G, Hader WJ. Endoscopic third ventriculostomy for treatment of adult hydrocephalus: Long-term follow-up of 163 patients. Neurosurg Focus. 2016. 41: E3
14. Jenkinson MD, Hayhurst C, Al-Jumaily M, Kandasamy J, Clark S, Mallucci CL. The role of endoscopic third ventriculostomy in adult patients with hydrocephalus. J Neurosurg. 2009. 110: 861-6
15. Jin JY, Yin FF, Tenn SE, Medin PM, Solberg TD. Use of the BrainLAB ExacTrac X-Ray 6D system in image-guided radiotherapy. Med Dosim. 2008. 33: 124-34
16. Kim J, Jin JY, Walls N, Nurushev T, Movsas B, Chetty IJ. Image-guided localization accuracy of stereoscopic planar and volumetric imaging methods for stereotactic radiation surgery and stereotactic body radiation therapy: A phantom study. Int J Radiat Oncol Biol Phys. 2011. 79: 1588-96
17. Koch D, Grunert P, Filippi R, Hopf N. Re-ventriculostomy for treatment of obstructive hydrocephalus in cases of stoma dysfunction. Minim Invasive Neurosurg. 2002. 45: 158-63
18. Lawrence YR, Li XA, El Naqa I, Hahn CA, Marks LB, Merchant TE. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010. 76: S20-7
19. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951. 102: 316-9
20. Luo G, Neimat JS, Cmelak A, Kirschner AN, Attia A, Morales-Paliza M. Margin of error for a frameless image guided radiosurgery system: Direct confirmation based on posttreatment MRI scans. Pract Radiat Oncol. 2017. 7: e223-1
21. Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010. 76: S36-41
22. Mugamba J, Stagno V. Indication for endoscopic third ventriculostomy. World Neurosurg. 2013. 79: S20e19-23
23. Oertel JM, Schroeder HW, Gaab MR. Third ventriculostomy for treatment of hydrocephalus: Results of 271 procedures. Neurosurg Q. 2006. 16: 24-31
24. Pan L, Dai JZ, Wang BJ, Xu WM, Zhou LF, Chen XR. Stereotactic Gamma thalamotomy for the treatment of parkinsonism. Stereotact Funct Neurosurg. 1996. 66: 329-32
25. Rahimian J, Chen JC, Rao AA, Girvigian MR, Miller MJ, Greathouse HE. Geometrical accuracy of the Novalis stereotactic radiosurgery system for trigeminal neuralgia. J Neurosurg. 2004. 101: 351-5
26. Sacko O, Boetto S, Lauwers-Cances V, Dupuy M, Roux FE. Endoscopic third ventriculostomy: Outcome analysis in 368 procedures. J Neurosurg Pediatr. 2010. 5: 68-74
27. Schroeder HW, Niendorf WR, Gaab MR. Complications of endoscopic third ventriculostomy. J Neurosurg. 2002. 96: 1032-40
28. Seung SK, Larson DA, Galvin JM, Mehta MP, Potters L, Schultz CJ. American college of radiology (ACR) and American society for radiation oncology (ASTRO) practice guideline for the performance of stereotactic radiosurgery (SRS). Am J Clin Oncol. 2013. 36: 310-5
29. Shin M, Kurita H, Sasaki T, Tago M, Morita A, Ueki K. Stereotactic radiosurgery for pituitary adenoma invading the cavernous sinus. J Neurosurg. 2000. 93: 2-5
30. Sicignano G, Losa M, del Vecchio A, Cattaneo GM, Picozzi P, Bolognesi A. Dosimetric factors associated with pituitary function after Gamma knife surgery (GKS) of pituitary adenomas. Radiother Oncol. 2012. 104: 119-24
31. Siomin V, Weiner H, Wisoff J, Cinalli G, Pierre-Kahn A, Saint-Rose C. Repeat endoscopic third ventriculostomy: Is it worth trying?. Childs Nerv Syst. 2001. 17: 551-5
32. Solberg TD, Medin PM, Mullins J, Li S. Quality assurance of immobilization and target localization systems for frameless stereotactic cranial and extracranial hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2008. 71: S131-5
33. Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003. 55: 1177-81
34. Verellen D, Soete G, Linthout N, van Acker S, de Roover P, Vinh-Hung V. Quality assurance of a system for improved target localization and patient set-up that combines real-time infrared tracking and stereoscopic X-ray imaging. Radiother Oncol. 2003. 67: 129-41
35. Vladyka V, Liscak R, Novotny J, Marek J, Jezkova J. Radiation tolerance of functioning pituitary tissue in gamma knife surgery for pituitary adenomas. Neurosurgery. 2003. 52: 309-16
36. Vulcu S, Eickele L, Cinalli G, Wagner W, Oertel J. Long-term results of endoscopic third ventriculostomy: An outcome analysis. J Neurosurg. 2015. 123: 1456-62
37. Woodworth G, McGirt MJ, Thomas G, Williams MA, Rigamonti D. Prior CSF shunting increases the risk of endoscopic third ventriculostomy failure in the treatment of obstructive hydrocephalus in adults. Neurol Res. 2007. 29: 27-31
38. Wurm RE, Erbel S, Schwenkert I, Gum F, Agaoglu D, Schild R. Novalis frameless image-guided noninvasive radiosurgery: Initial experience. Neurosurgery. 2008. 62: A11-17
39. Yadav YR, Parihar V, Pande S, Namdev H, Agarwal M. Endoscopic third ventriculostomy. J Neurosci Rural Pract. 2012. 3: 163-73
40. Yahya S, Heyes G, Nightingale P, Lamin S, Chavda S, Geh I. Linear accelerator radiosurgery for arteriovenous malformations: Updated literature review. J Clin Neurosci. 2017. 38: 91-5