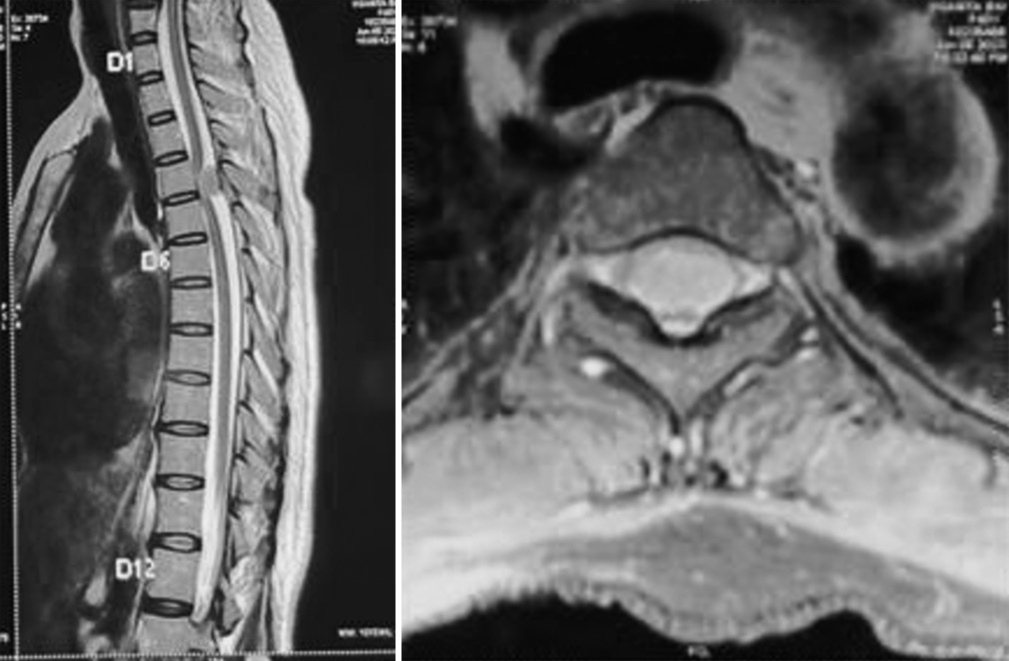
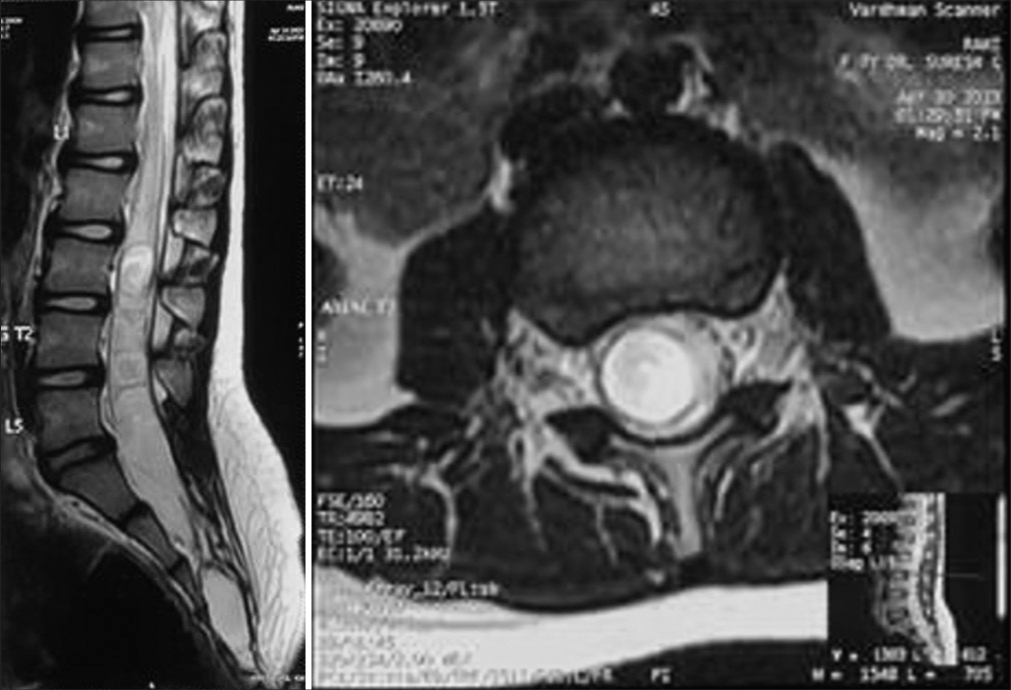
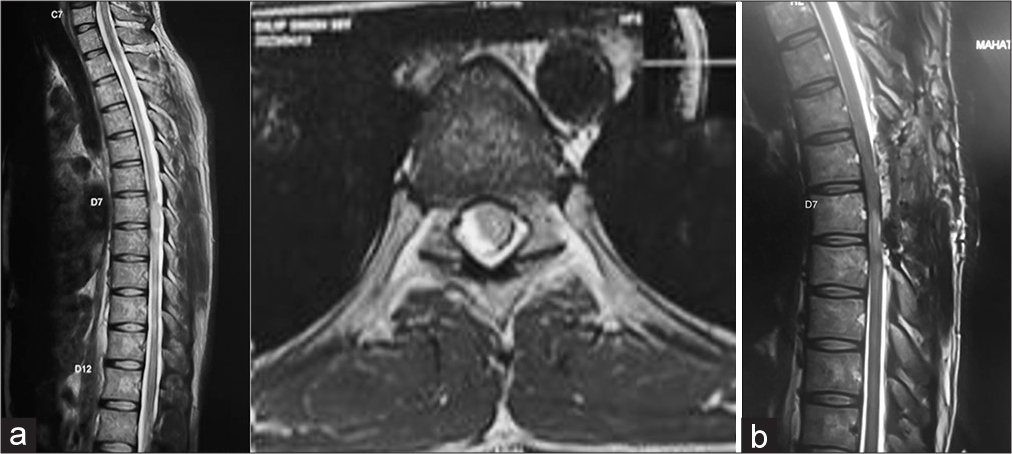
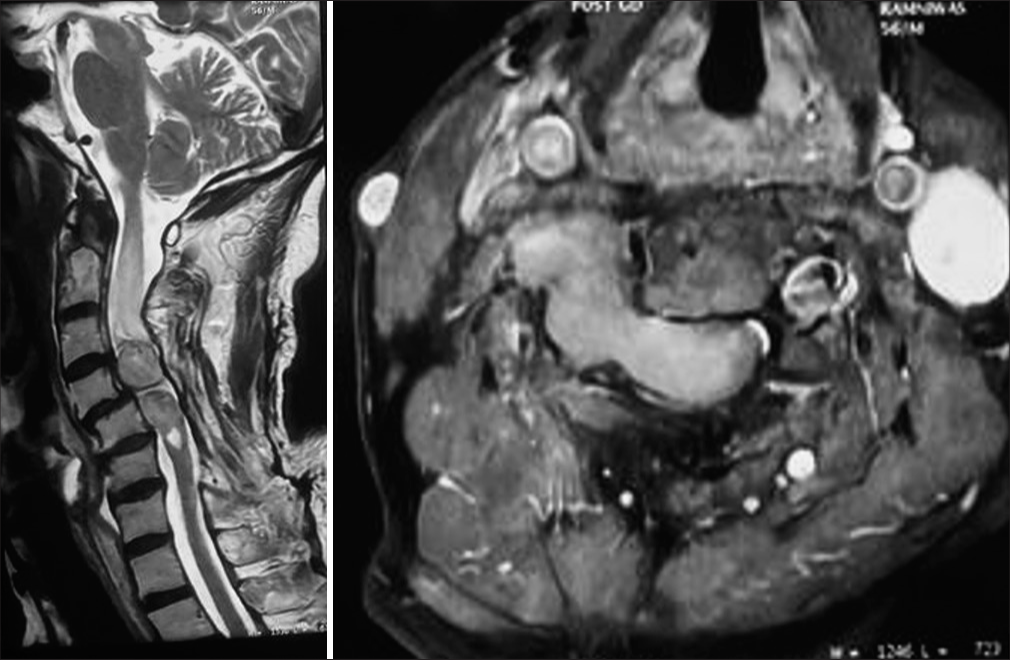
- Department of Neurosurgery, Mahatma Gandhi University of Medical Sciences and Technology, Jaipur, Rajasthan, India.
Correspondence Address:
Anmol Singh Randhawa, Department of Neurosurgery, Mahatma Gandhi University of Medical Sciences and Technology, Jaipur, Rajasthan, India.
DOI:10.25259/SNI_689_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anmol Singh Randhawa, Anurag Srivastava, Shiteez Agrawal, Jitendra Singh Verma, Bhawani Shankar Sharma, Tshering Dorjee Sherpa. Functional outcomes in intradural extramedullary spinal tumors. 05-Apr-2024;15:114
How to cite this URL: Anmol Singh Randhawa, Anurag Srivastava, Shiteez Agrawal, Jitendra Singh Verma, Bhawani Shankar Sharma, Tshering Dorjee Sherpa. Functional outcomes in intradural extramedullary spinal tumors. 05-Apr-2024;15:114. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12849
Abstract
Background: Intradural extramedullary (IDEM) spinal cord tumors account for approximately two-thirds of benign intraspinal neoplasms. These are amenable to gross total excision but can have variable functional outcomes, which plays a key role in assessing their impact on a patient’s quality of life. Understanding the functional outcomes associated with these tumors is crucial for healthcare professionals to devise appropriate treatment plans and provide comprehensive care.
Methods: In this study, we retrospectively reviewed the outcomes of 130 patients with IDEM tumors who underwent surgery in the past six years between January 2017 and December 2022 at a single institution. Patient demographics, symptoms, and tumor characteristics (anatomical and pathological) in all operated spinal IDEM tumors were analyzed. The neurological findings obtained during the preoperative stage and the postoperative follow-up were evaluated according to the Frankel grading. The back pain was assessed using the Denis pain scale (DPS).
Results: The age range, gender distribution, presentation, histopathology, and tumor characteristics were analyzed. The histopathological outcomes of the study were as follows: 56 cases of schwannoma, 37 cases of meningiomas, 16 patients of neurofibroma, six cases of epidermoid cyst, five cases each of ependymoma and dermoid cyst, three cases of arachnoid cyst, two cases of metastasis, and one case of paraganglioma. Pain was the most common symptom (38.5%), followed by weakness in limbs (31.5%), paresthesia/numbness (22.3%), and sphincter disturbance (7.7%). Complete total resection was seen in 93% of cases, with 7% undergoing subtotal excision. The complications encountered were – four cases of surgical site infection and one case each of cerebrospinal fluid leak, pseudomeningocele, and epidural hematoma. In our series, 49.3% of patients had significantly good improvement in functional outcomes as per improvement in Frankel score, and 43% of patients had good functional improvement. Significant functional improvement was noted at immediate postoperative follow-up, 2-week follow-up, and six-month follow-up periods. Reoccurrence was seen in 7 cases (5.4%). The DPS score mean values showed a significant decrease over the follow-up duration as compared to preoperative mean values. Significantly poor outcome was seen in IDEM tumours present anteriorly.
Conclusion: The IDEM tumors are usually benign and are readily detected by contrast-enhanced magnetic resonance imaging scans. These have variable functional outcomes in different centers. Assessing this functional outcome is an essential aspect of managing IDEM spinal tumors. It was observed through our study that the ventral location of the tumor, thoracic tumors, and poor preoperative neurological status of the patient correspond with poorer postoperative functional outcomes. Furthermore, a significant decrease in the pain symptoms with improvement of Frankel score was seen postoperatively, thus this being suggestive of a significant improvement of functional outcome after surgery. This study helps to conclude that the morbidity associated with the resection of IDEM tumors is not as significant as originally thought to be.
Keywords: Denis pain scale, Frankel grade, Functional outcome, Intradural extramedullary spine tumors, Nerve monitoring
INTRODUCTION
Spinal intradural extramedullary (IDEM) tumors are uncommon, with a reported incidence of 3–10/100,000 people, and account for two-thirds of all primary intraspinal neoplasms.[
Over the years, there has been no significant change in the clinical symptoms and pathology of IDEM tumors. However, there have been dramatic improvements in the diagnosis and treatment with the advances in radiological and surgical techniques. Despite advances in operative techniques and neuroimaging, the morbidity associated with the resection of IDEM tumors continues to be significant.[
The study aims to analyze patient demographics, symptoms, and tumor characteristics (anatomical and pathological) in all operated spinal IDEM tumors and to correlate their preoperative neurological status and postoperative functional outcomes.
MATERIALS AND METHODS
A retrospective observational study was carried out over 6 years at the Department of Neurosurgery, Mahatma Gandhi University of Medical Sciences and Technology, Jaipur, India. The study had a sample size of 130 patients with IDEM spine tumors who underwent microsurgical resection of the tumor.
Inclusion criteria
All patients undergoing surgery for solitary spinal IDEM tumors (primary/recurrent) during the study duration were included in the study.
Exclusion criteria
Patients with illness-limiting surgical interventions were excluded from the study. Patients who were lost to follow-up or those who refused repeat magnetic resonance imaging (MRI) scans were also excluded from the study.
Methods
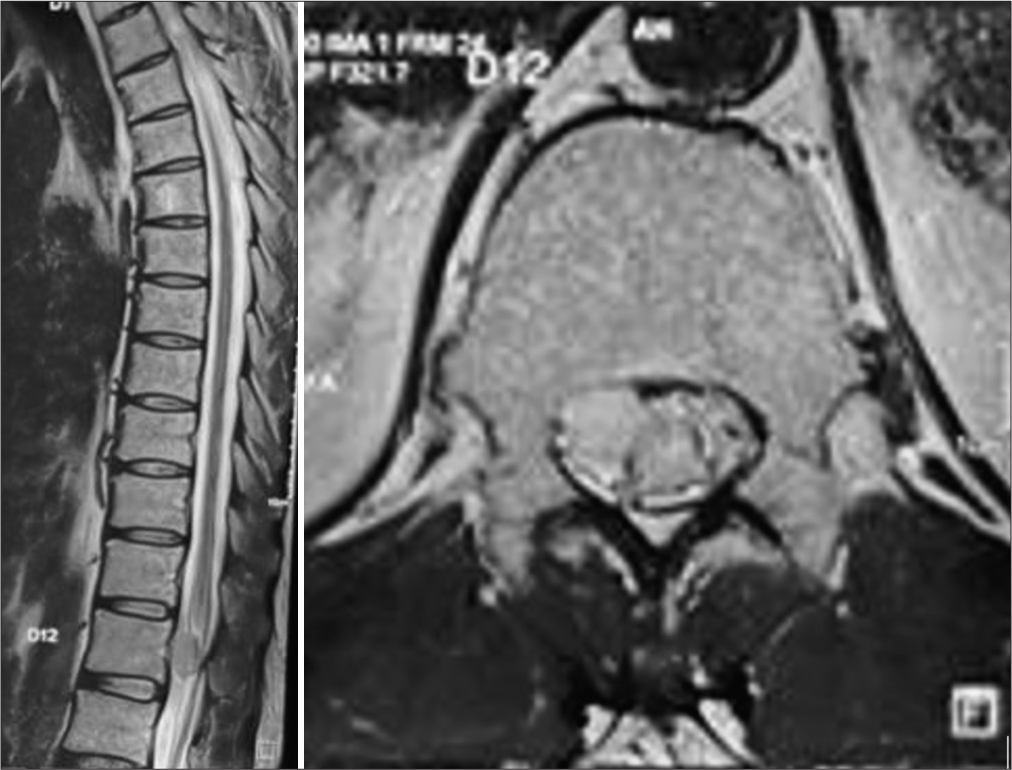
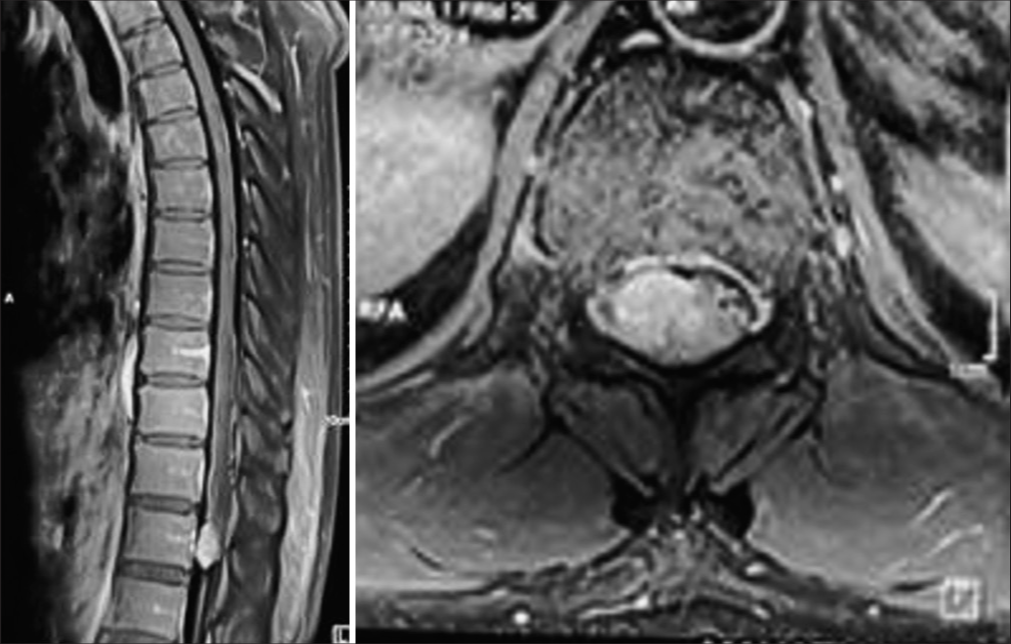
The registration data, duration of symptoms, and nature of complaints were recorded. Preoperative pain severity was assessed using the Denis pain scale (DPS). Preoperative functional status was recorded using the Frankel grading scales. Written and informed consent was obtained from all patients and their representatives regarding their willingness to be a part of the study and the follow-up process. All patients underwent a contrast-enhanced MRI spine scan during the initial surgical workup. The location of the tumor on sagittal and axial MRI images was recorded preoperatively. After relevant investigations, patients were electively posted for laminectomy and gross total excision of the tumor under general anesthesia.
Surgical technique
In this study, a microsurgical facet-sparing posterior approach was used in all the cases. After laminectomy and exposure of the dura, the dura was opened, and the arachnoid was cut to mobilize the tumor. Tumors are initially decompressed using a Cavitronic ultrasonic aspirator. No attempt should be made to dissect the outside of the tumor until it has been cored out from within and only a thin rim of the tumor capsule is left, as trying to deliver the tumor without reducing its bulk can result in injury to the adjacent normal neural structures. Furthermore, the origin of the tumor on the dura was coagulated by a bipolar coagulator. In the case of schwannoma, the affected root was usually coagulated and cut. For meningiomas, the involved dura was either excised or, most often, coagulated completely. After the tumor excision was performed using an operating microscope, the dura was closed primarily with a 4-0 Proline. Closed wound suction drainage was performed in all the cases. Patients ambulated within a few hours after surgery and are usually discharged on the 2nd or 3rd postoperative day after drain removal. In patients with meningiomas in whom the dural defect could not be repaired, a thoracolumbar fascia or a fat graft was layered over the defect and covered with a gelatin sponge.
Furthermore, they were placed on bed rest in the prone position with a wound drain in place for 4–5 days. They were subsequently mobilized, and in most cases, we were able to avoid a cerebrospinal fluid (CSF) leak using this technique. The aim of surgery while operating on meningiomas was to achieve Simpson’s Grade 2.
Intraoperative monitoring
Motor evoked potentials (MEPs)[
MEPs were elicited using electric transcranial stimulation (NIM-eclipse®). Corkscrew electrodes were placed in C1–C2 positions (10–20 international EEG system) using C1 or C2 as an anode for the right or left limbs, respectively. A series of 5–7 stimuli (pulse width 75–500 µs, 250–500 Hz, 75–750 V) were delivered in order to obtain at least a stable muscular response for each studied limb with 100 µV amplitude.[
Somatosensory evoked potentials (SSEPS) was not used in our institute as per our institutional protocol.
Following surgery, the primary outcome assessment was done. Patients were graded postoperatively on the Frankel scale immediately postoperatively, at a two-week follow-up, and six-month follow-up for the evaluation of functional status. The tumor histopathology report was recorded. A postoperative MRI scan was done at six months, whenever required, in patients who underwent subtotal resection or who did not improve symptomatically or functionally.
In the secondary outcome assessment, patients were assessed for remission in pain postoperatively using the DPS at immediate postoperative period, two week, and six months. Complications of the surgical procedure were recorded.
Frankel grading
A: Complete (no sensory or motor function is preserved)
B: Incomplete (Sensory, but no motor function is preserved below the neurological level)
C: Incomplete (Motor function is preserved below the neurological level, and the majority of key muscles below the neurological level have a muscle power grade of <3)
D: Incomplete (Motor function is preserved below the neurological level, and the majority of key muscles below the neurological level have a muscle power grade of >3)
E: Normal (sensory and motor function is normal).[
DPS
P1: No pain
P2: Occasional minimal pain; no need for medication
P3: Moderate pain, occasionally medications; no interruption of work or activities of daily living
P4: Moderate to severe pain, occasionally absent from work; significant changes in activities of daily living
P5: Constant, severe pain; chronic pain medications.[
The statistical analysis was done using the Statistical Package for the Social Sciences (SPSS Statistics) version 22 (IBM Corp., Armonk, USA). Paired Student’s t-test, multivariate analysis of variance, and Mann–Whitney U-test were performed on the data.
We defined a “Significant improvement” as an improvement of ≥2 Frankel grade. Patients with an improvement of <2 Frankel grade or unchanged grade were labeled as having “good improvement.” The patients showing deterioration of Frankel score were considered to have a “poor outcome” [
RESULTS
The study revealed an even distribution of IDEMS (IDEM spinal tumors) between both genders, with no notable gender predisposition to the condition. The highest number of cases was observed within the 21–30 age range. The DPS was employed to evaluate pain levels both before and after IDEM tumor excision surgery, indicating a substantial reduction in postoperative pain.
The most frequently reported preoperative symptoms included dull aching/radicular pain, paraparesis/limb weakness, paresthesia/numbness, and sphincter dysfunction. The majority of IDEMS cases were situated posterior to the spinal cord, followed by posterolateral and anterior positions. The most common tumor location was in the dorsal region, with the lumbar, thoracolumbar, cervical, cervicothoracic, and lumbosacral regions following in decreasing order.
Nerve sheath tumors were the predominant type, with schwannoma being the most prevalent, followed by meningiomas, neurofibroma, epidermoid cysts, ependymoma, dermoid cysts, arachnoid cysts, metastasis, and paraganglioma.
Demographic details
Sex
There was an equal distribution of IDEMS among both genders, with no gender showing a significant predisposition for the disease [
Age
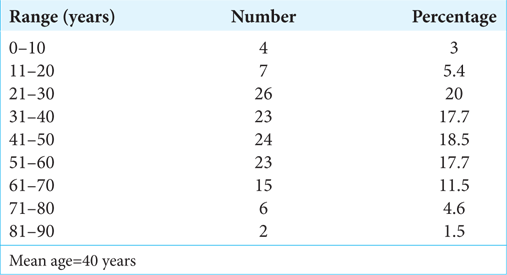
Maximum cases were seen in the age group range of 21–30 years (20%)[
Preoperative and postoperative DPS
DPS was used to grade the degree of pain preoperatively as well as postoperatively, and there was shown to be a highly significant decrease in pain in patients operated for IDEM tumor excision surgery [
Preoperative symptoms
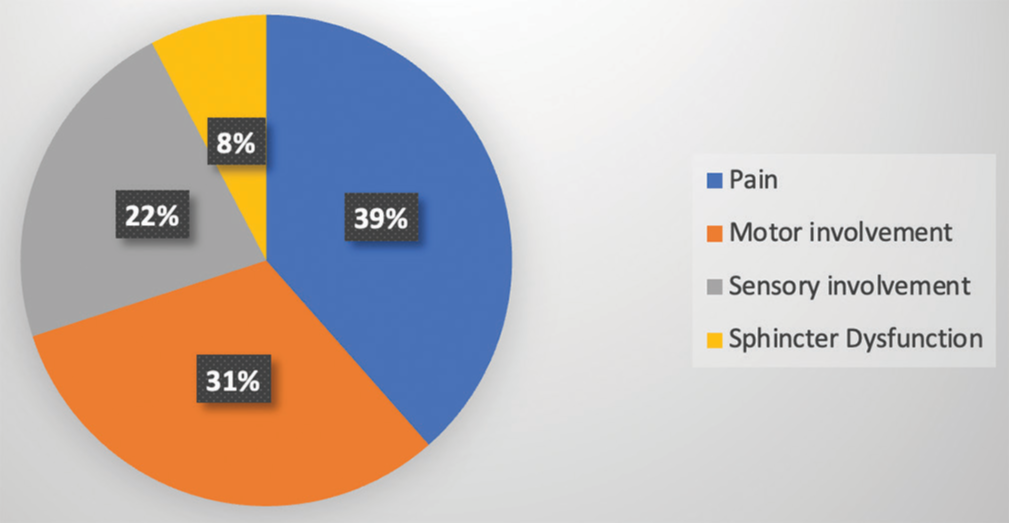
The most common preoperative complaint was dull aching/radicular pain in 50 patients (38.5%), followed by paraparesis/weakness of limbs in 41 patients (31.5%) and paresthesia/numbness in 29 patients (22.3%). Sphincter dysfunction was present in 10 patients (7.7%) [
Axial distribution of tumors
Maximum cases of IDEMS were seen to be distributed posteriorly to the cord (50%), with 30.8% being present posterolaterally and only 19.2% was seen to be present anteriorly to the cord [
Sagittal distribution of tumors
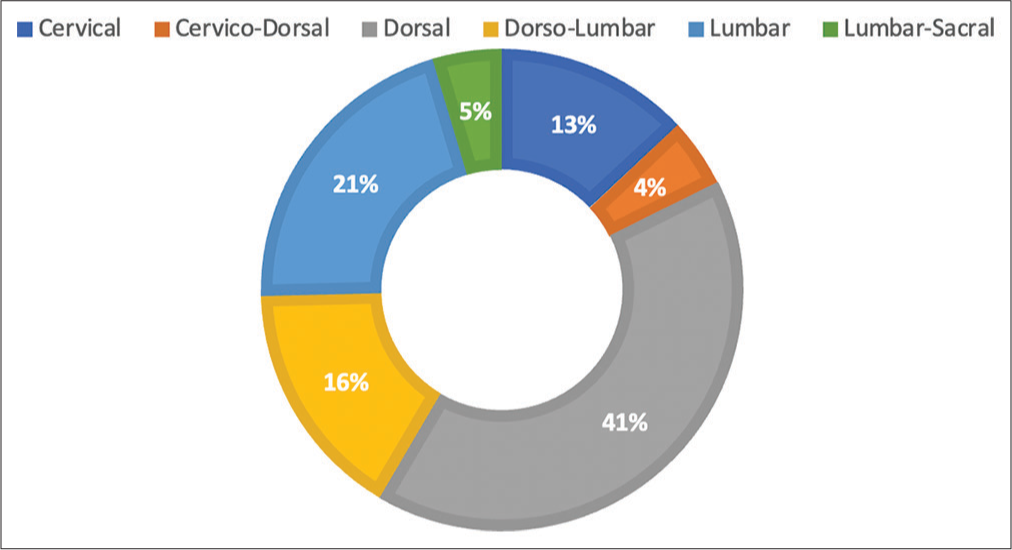
The most common location of the tumor was the dorsal region (40.7%), followed by 20.7% in the lumbar location, 16.1% at the thoracolumbar location, 13% in the cervical region, and 4.6% in the cervicothoracic and lumbosacral locations[
Pathological types
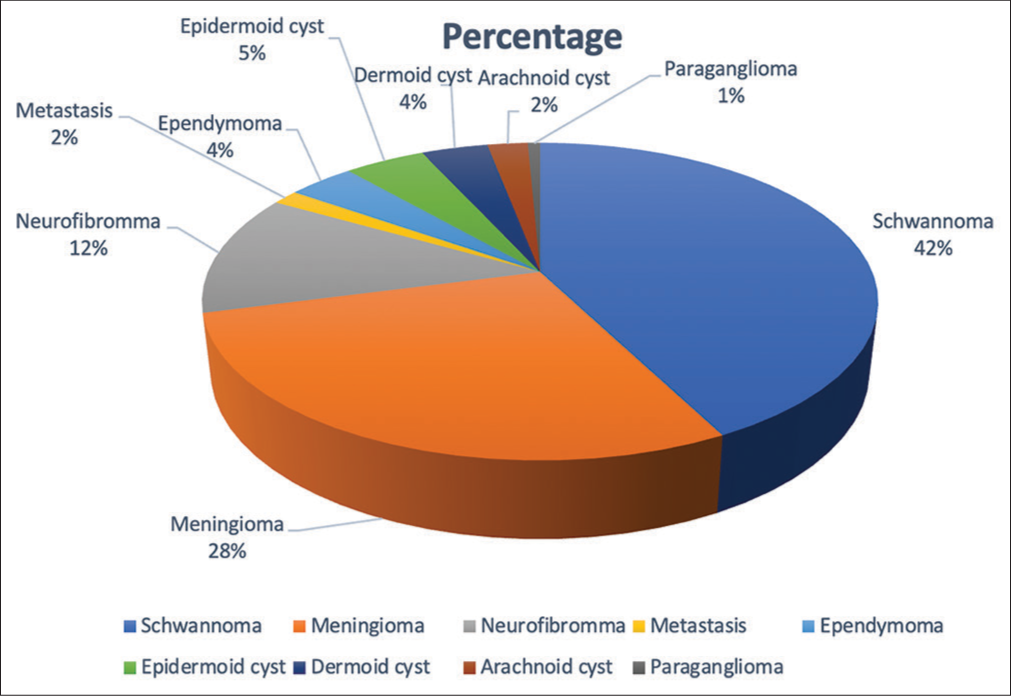
Nerve sheath tumor was the most common type. There were 56 cases of schwannoma, 37 cases of meningiomas, 16 patients of neurofibroma, six cases of epidermoid cyst, five cases each of ependymoma and dermoid cyst, three cases of arachnoid cyst, two cases of metastasis, and only one case of paraganglioma [
Follow-up duration
All patients were followed up immediately postoperatively, at two weeks and six months, and repeat MRI was advised after six months of surgery whenever required in patients who underwent subtotal resection or who did not improve symptomatically or functionally. Regular follow-up of all patients could not be done after six months. In our study, residual/recurrent lesion was seen in seven cases. Out of these seven cases, two were metastasis, one each of schwannoma, ependymoma, epidermoid, dermoid, and meningioma. Out of these, one case of ependymoma, schwannoma, epidermoid, and dermoid underwent redo surgery. Of the other three cases, one recurrent case of meningioma who refused redo surgery and two other cases of metastasis were all considered for adjuvant radiotherapy.
Surgical treatment
One hundred and thirty patients underwent laminectomy one level above, one level below, and at the level of the tumor, and fixation was required in two patients.
The tumors located posterior or dorsal to the cord were resected with a posterior approach with laminectomy. The posterolateral-located tumors and the anteriorly situated tumors also underwent gross excision with gentle cord rotation through a posterior approach. All cases underwent resection through a posterior approach.
Nerve monitoring was used in 84 cases, out of which 80 cases had good outcomes and 4 (5%) cases had poor outcomes. However, out of the 46 cases where nerve monitoring was not used, 6 had poor outcomes (13%).
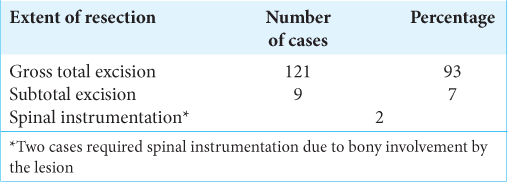
One hundred and twenty-one patients (93%) underwent microsurgical gross total excision of the tumor. However, nine patients underwent subtotal excision of tumor 7% (9 out of 130 patients) due to severe adhesions [
Morbidity and mortality
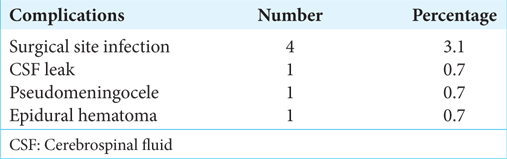
The complications encountered in the postoperative period were through CSF leak from the wound site in a case of thoracic neurofibroma, surgical site infection in four cases, epidural hematoma in one case of thoracic schwannoma, and pseudo meningocele in a case of lumbar dermoid cyst. There was no incidence of spinal instability, meningitis, or operative mortality [
Functional outcome
In our series, 64/130 (49.3%) patients had significant improvement of ≥2 grades on Frankel score, and 56/130 (43%) patients had good improvement as per Frankel score at the time of last follow-up, and therefore, 120/130 (92.3%) patients had “good outcome.” However, 10 (7.7%) patients had no improvement in postoperative Frankel grade and had “poor outcomes.”
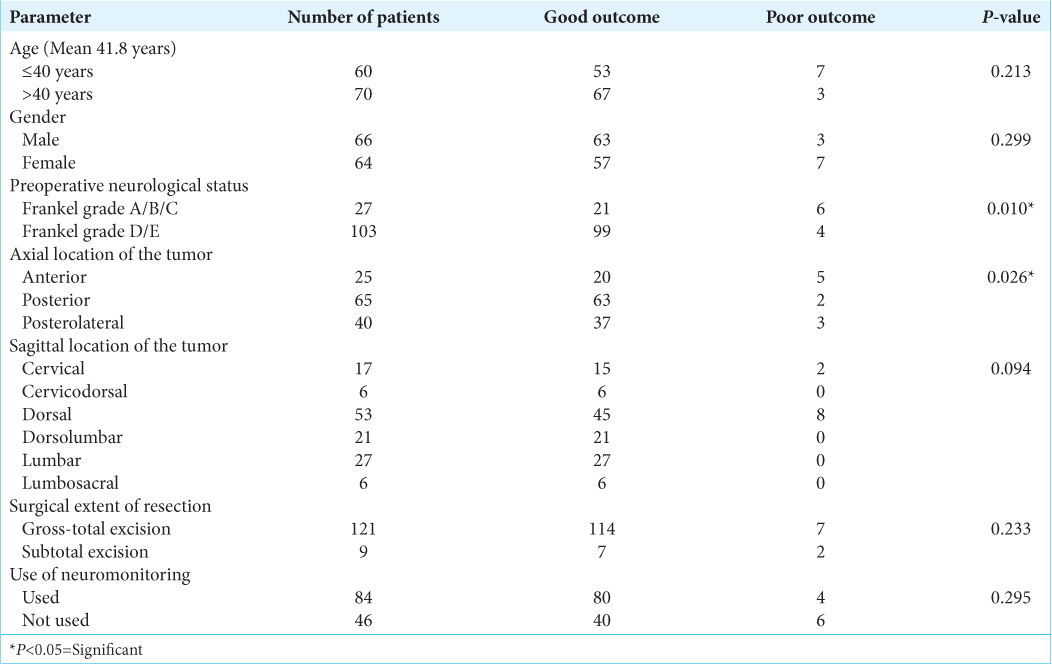
It was seen that there was also a significant relationship with the preoperative neurological status of the patient, with the patients categorized under Frankel grade A/B/C showing significantly poor postoperative functional outcome, as compared to the patients categorized under Frankel D/E. There was a significant difference in the axial location of the tumor affecting the postoperative functional outcome also, with the tumors being present anterior to the cord showing a significantly poor outcome as compared to the ones being present posteriorly. It was seen that the more proximal the location of a tumor was and the more ventral the tumor was to the spinal cord, the worse the surgical outcome was. The DPS mean values showed a significant decrease over the follow-up duration as compared to preoperative mean values [
DISCUSSION
IDEM tumors are usually benign and account for two-thirds of primary spinal tumors. Studies indicate that five females and three males out of 1,000,000 people are affected by primary spinal tumors each year,[
The age distribution in our study showed a peak incidence of 21–30 years. This was unlike the age group reported by Seppälä et al.[
The gender distribution in our study was 50.8% male and 49.2% female. This was comparable to rates reported by Seppälä et al.,[
As per Nitter,[
In our study, nerve sheath tumors formed the largest histopathological group with 55 (42.3%) patients, followed by 37 (28.4%) patients with meningiomas, and the third most common group of 16 (12.3%) patients with neurofibromas. The histopathological distribution of IDEM tumors in our study conformed to observations made by Arora and Kumar,[
Meningiomas account for 25–46% of all primary intraspinal neoplasms, and spinal meningiomas are only 7.5–12.5% of all meningiomas because most meningiomas are found in the brain.[
In our study, there is an association of the axial and sagittal tumor location with functional outcome; that is, thoracic tumors and ventral tumors have shown poorer outcomes as compared to other locations. This was similar to other literature where the thoracic and ventral locations of the tumor were noted to have poor postoperative outcomes.[
With regard to the factors that influence the prognosis, it has been seen that the more proximal the location of a tumor is and the more ventral the tumor is to the spinal cord, the worse the surgical outcome. This result in our study has been similar to the results shown by Slin’ko and Al-Qashqish, Júnior et al., and Subramanian et al.[
In our study, there was a significant decrease in the DPS score observed over the follow-up period, which was suggestive of a highly significant decrease in the pain symptoms postoperatively.
Furthermore, a significant improvement in the mean Frankel score was seen in our study and was suggestive that there was a significant improvement in functional outcomes after surgery.
In this study, a posterior approach was used in all the cases regardless of the location of a tumor in and relative to the spinal cord. According to the literature, 31% of the tumors are located ventral to the spinal cord[
Postoperative motor deficits following neurosurgical procedures result in significant morbidity and mortality rates as well as increased medical costs associated with the extended length of stay and rehabilitation.[
In recent years, muscle MEPs (mMEPs) are also monitored. mMEPs recorded through EMG electrodes in the musculature of the upper and lower limbs after motor cortex stimulation by scalp electrodes aim to obviate this problem. Many neurosurgeons now advocate mMEP monitoring for all spinal surgery since they better predict good postoperative motor outcomes than the use of SSEPs alone. In addition to this predictive power, mMEP data recording benefits from a high temporal resolution; the data may be updated on the order of seconds, providing the surgeon with “real time” information regarding possible surgical trauma. For these reasons, SSEPs are not routinely used during IDEM surgery in our center. However, recent studies have reported the benefits of combining mMEP with SSEP monitoring.[
According to the previous studies, most of the neurological symptoms improved postoperatively in most of the cases, but partial improvements, no improvement, and deterioration were also observed in some cases. However, surgical intervention should still be recommended for all IDEM tumors regardless of the prognostic factors because Frankel’s grade improved postoperatively significantly for the majority of the cases in our study regardless of the prognostic factors.
CSF leak from the wound site was seen in a case of thoracic neurofibroma, which was managed medically. Surgical site infections were noted in four cases, which were managed with oral antibiotics. A case of lumbar dermoid cyst developed pseudomeningocele after three weeks of surgery, which was managed conservatively with compression dressings and sequential aspirations. Another patient had to undergo re-exploration for evacuation of epidural hematoma on postoperative day 1, in which the dural defect was repaired with a fascia graft with fibrin glue reinforcement.
The postoperative recurrence rate of IDEM spinal cord tumors varied between 5% and 16% in the literature.[
CONCLUSION
The IDEM tumors are usually benign and are readily detected by contrast-enhanced MRI scans. These have variable functional outcomes in different centers. Assessing this functional outcome is an essential aspect of managing IDEM spinal tumors. These tumors can have significant implications for a patient’s neurological function, impacting sensory abilities, motor abilities, and bladder or bowel control. Improving functional outcomes not only enhances the quality of life for individuals affected by these tumors but also helps guide medical interventions, rehabilitation efforts, and supportive services. With a multidisciplinary approach and a focus on functional outcomes, healthcare providers can strive to optimize patient outcomes and promote overall well-being for those living with IDEM spinal tumors.
It was observed through our study that the thoracic location of the tumor and the poor preoperative neurological status of the patient correspond with poorer postoperative functional outcomes. There was a significant difference in the axial location of the tumor, affecting the postoperative functional outcome also, with the tumors being present anterior to the cord showing a significantly poor outcome as compared to the ones being present posteriorly. Furthermore, a significant decrease in the pain symptoms with the improvement of Frankel score was seen postoperatively. Thus this being suggestive of a significant improvement of functional outcome after surgery.
Ethical approval
The Institutional Review Board Approval is not required.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abul-Kasim K, Thurnher MM, McKeever P, Sundgren PC. Intradural spinal tumors: Current classification and MRI features. Neuroradiology. 2008. 50: 301-14
2. Ahn DK, Jung KW, Lee S, Choi DJ, Park JS, Cha SG. A 6-year observation of multiple spinal schwannomas before excision-1 case report. J Korean Soc Spine Surg. 2003. 10: 277-82
3. Ahn DK, Park HS, Choi DJ, Kim KS, Kim TW, Park SY. The surgical treatment for spinal intradural extramedullary tumors. Clin Orthop Surg. 2009. 1: 165-72
4. Arora R, Kumar R. Spinal tumors: Trends from Northern India. Asian J Neurosurg. 2015. 10: 291-7
5. Asazuma T, Toyama Y, Watanabe M, Suzuki N, Fujimura Y, Hirabayashi K. Clinical features associated with recurrence of tumours of the spinal cord and cauda equina. Spinal Cord. 2003. 41: 85-9
6. Choi I, Hyun SJ, Kang JK, Rhim SC. Combined muscle motor and somatosensory evoked potentials for intramedullary spinal cord tumour surgery. Yonsei Med J. 2014. 55: 1063-71
7. D’Ercole M, D’Alessandris QG, Di Domenico M, Burattini B, Menna G, Izzo A. Is there a role for intraoperative neuromonitoring in intradural extramedullary spine tumors? results and indications from an institutional series. J Pers Med. 2023. 13: 1103
8. Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: A review focus on the corticospinal tracts. Clin Neurophysiol. 2008. 119: 248-64
9. Denis F, Armstrong GW, Searls K, Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit a comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984. 189: 142-9
10. El-Mahdy W, Kane PJ, Powell MP, Crockard HA. Spinal intradural tumours: Part I-extramedullary. Br J Neurosurg. 1999. 13: 550-7
11. Fernandes RL, Lynch JC, Welling L, Gonçalves M, Tragante R, Temponi V. Complete removal of the spinal nerve sheath tumors. Surgical technics and results from a series of 30 patients. Arq Neuropsiquiatr. 2014. 72: 312-7
12. Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Spinal Cord. 1969. 7: 179-92
13. Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH. Spinal meningiomas: Surgical management and outcome. Neurosurg Focus. 2003. 14: e2
14. Govind M, Radheyshyam M, Achal S, Ashok G. Intradural extramedullary spinal cord tumors: A retrospective study at tertiary referral hospital. Roman Neurosurg. 2016. 30: 106-12
15. Helseth A, Mørk SJ. Primary intraspinal neoplasms in Norway, 1955 to 1986: A population-based survey of 467 patients. J Neurosurg. 1989. 71: 842-5
16. Júnior EC, Dantas F, Caires AC, Cariri GA, Reis MT, Botelho RV. Evaluation of surgically treated primary spinal cord tumors in a single Brazilian institution: A case series study of 104 patients. Cureus. 2022. 14: e23408
17. Kankane VK, Jaiswal G, Gupta TK. Surgical outcome of intradural extramedullary tumors: Single institutional experience-assessment using Frankel grading. J Spinal Surg. 2015. 2: 118-24
18. Kim P, Ebersold MJ, Onofrio BM, Quast LM. Surgery of spinal nerve schwannoma: Risk of neurological deficit after resection of involved root. J Neurosurg. 1989. 71: 810-4
19. Kothbauer K, Deletis V, Epstein FJ. Intraoperative spinal cord monitoring for intramedullary surgery: An essential adjunct. Pediatr Neurosurg. 1997. 26: 247-54
20. Narayan S, Rege SV, Gupta R, Rege S. Clinicopathological study of intradural extramedullary spinal tumors and its correlation with functional outcome. Cureus. 2021. 13: e15733
21. Nitter K. Spinal meningiomas, neurinomas, and neurofibromas and hourglass tumors. Handb Clin Neurol. 1976. 20: 177-322
22. Parsa AT, Lee J, Parney IF, Weinstein P, McCormick PC, Ames C. Spinal cord and intradural-extraparenchymal spinal tumors: Current best care practices and strategies. J Neurooncol. 2004. 69: 291-318
23. Pelosi L, Lamb J, Grevitt M, Mehdian SM, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002. 113: 1082-91
24. Prevedello DM, Koerbel A, Tatsui CE, Truite L, Grande CV, Ditzel LF. Prognostic factors in the treatment of the intradural extramedullary tumors: A study of 44 cases. Arq Neuropsiquiatr. 2003. 61: 241-7
25. Saito T, Arizono T, Maeda T, Terada K, Iwamoto Y. A novel technique for surgical resection of spinal meningioma. Spine. 2001. 26: 1805-8
26. Seppälä MT, Haltia MJ, Sankila RJ, Jääskeläinen JE, Heiskanen O. Long-term outcome after removal of spinal schwannoma: A clinicopathological study of 187 cases. J Neurosurg. 1995. 83: 621-6
27. Slin’ko EI, Al-Qashqish II. Intradural ventral and ventrolateral tumors of the spinal cord: Surgical treatment and results. Neurosurg Focus. 2004. 17: ECP2
28. Solero CL, Fornari M, Giombini S, Lasio G, Oliveri G, Cimino C. Spinal meningiomas: Review of 174 operated cases. Neurosurgery. 1989. 25: 153-60
29. Song KW, Shin SI, Lee JY, Kim GL, Hyun YS, Park DY. Surgical results of intradural extramedullary tumors. Clin Orthop Surg. 2009. 1: 74-80
30. Subramanian A, Nair BR, Rajshekhar V. Functional outcomes and temporal profile of recovery in patients with intradural extramedullary spinal cord tumors with poor nurick grade. World Neurosurg. 2021. 146: e691-700
31. Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: Intraoperative changes and postoperative outcome. J Neurosurg. 2006. 105: 675-81