- Department of Clinical and Translational Research, The George Washington University School of Medicine and Health Sciences, Washington, DC, United States
- Department of Clinical and Translational Science, University of Massachusetts T.H. Chan School of Medicine, Worcester, United States
- Department of Inpatient Psychiatry, John F. Kennedy Medical Center, West Palm Beach, Florida, United States
Correspondence Address:
William Rienas, Department of Clinical and Translational Research, The George Washington University School of Medicine and Health Sciences, Washington, DC, United States.
DOI:10.25259/SNI_569_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: William Rienas1, Renxi Li1, SeungEun Lee1, Lianne Ryan2, Christopher Rienas3. Functionally dependent status is an independent predictor for worse perioperative outcomes following craniotomy for aneurysmal subarachnoid hemorrhage. 13-Sep-2024;15:333
How to cite this URL: William Rienas1, Renxi Li1, SeungEun Lee1, Lianne Ryan2, Christopher Rienas3. Functionally dependent status is an independent predictor for worse perioperative outcomes following craniotomy for aneurysmal subarachnoid hemorrhage. 13-Sep-2024;15:333. Available from: https://surgicalneurologyint.com/surgicalint-articles/13089/
Abstract
Background: Aneurysmal subarachnoid hemorrhage (aSAH) is a medical emergency, and functional status is often a predictor of adverse outcomes perioperatively. Patients with different functional statuses may have different perioperative outcomes during surgery for aSAH. This study retrospectively examines the effect of functional status on specific perioperative outcomes in patients receiving craniotomy for aSAH.
Methods: Patients with aSAH who underwent neurosurgery were identified using International Classification of Diseases (ICD) codes (ICD10, I60; ICD9, 430) in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database from 2005 to 2021. Subjects were stratified into two study groups: functionally dependent and functionally independent, based on their documented functional status on NSQIP. Significant preoperative differences were present between groups so a multivariable regression was performed between functionally dependent and independent patients. The 30-day perioperative outcomes of the two groups were compared. Perioperative outcomes included death, major adverse cardiovascular events (MACEs), cardiac complications, stroke, wound complications, renal complications, sepsis, clot formation, pulmonary complications, return to the operating room, operation time >4 h, length of stay longer than 7 days, discharge not to home, and bleeding.
Results: For aSAH patients receiving craniotomy repair, functionally dependent patients had significantly greater rates of MACE, cardiac complications, sepsis, pulmonary complications, and discharge not to home compared to functionally independent patients.
Conclusion: This study shows specific perioperative variables influenced by dependent functional status when treating aSAH through craniotomy, thus leading to a more complicated postoperative course. Additional research is needed to confirm these findings among the specific variables that we analyzed.
Keywords: Aneurysmal subarachnoid hemorrhage, Craniotomy, Dependent functional status, Independent functional status
INTRODUCTION
Subarachnoid hemorrhage (SAH) is characterized by the accumulation of blood in the subarachnoid space, located between the arachnoid and pia mater. The subarachnoid space is comprised of cerebrospinal fluid and blood vessels that may rupture in a hemorrhagic event. Although trauma is a common cause of SAH, non-traumatic SAH events mainly are a result of a ruptured aneurysm.[
Functional status reflects one’s health status as it refers to an individual’s ability to fulfill daily activities and maintain well-being.[
An aSAH is a medical emergency that warrants urgent treatment, and therefore, it is important to seek medical care early.[
MATERIALS AND METHODS
This retrospective study derived from ACS-NSQIP data does not require Institutional Review Board approval.
Data source
In this study, aSAH patients who underwent neurosurgery were identified using the international classification of diseases (ICD), 9th/10th Revision (ICD10, I60; ICD9, 430) in the ACS-NSQIP database from 2005 to 2021. In addition, patients who underwent craniotomy treatment by Current Procedural Terminology (CPT, 61697, 61700, 61698, 61702, 61703, 61705, 61312, and 61313) were identified in the ACSNSQIP database from 2005 to 2021. Patients were grouped into either DFS or IFS based on their designation in ACSNSQIP. Patients who were able to conduct all activities of daily living were classified as functionally independent, and those who could not be classified as functionally dependent.
ACS-NSQIP database, which includes over 400 sites nationwide, the database is used for quality control and monitors 30-day post-operative outcomes by the American College of Surgeons.
Preoperative variables
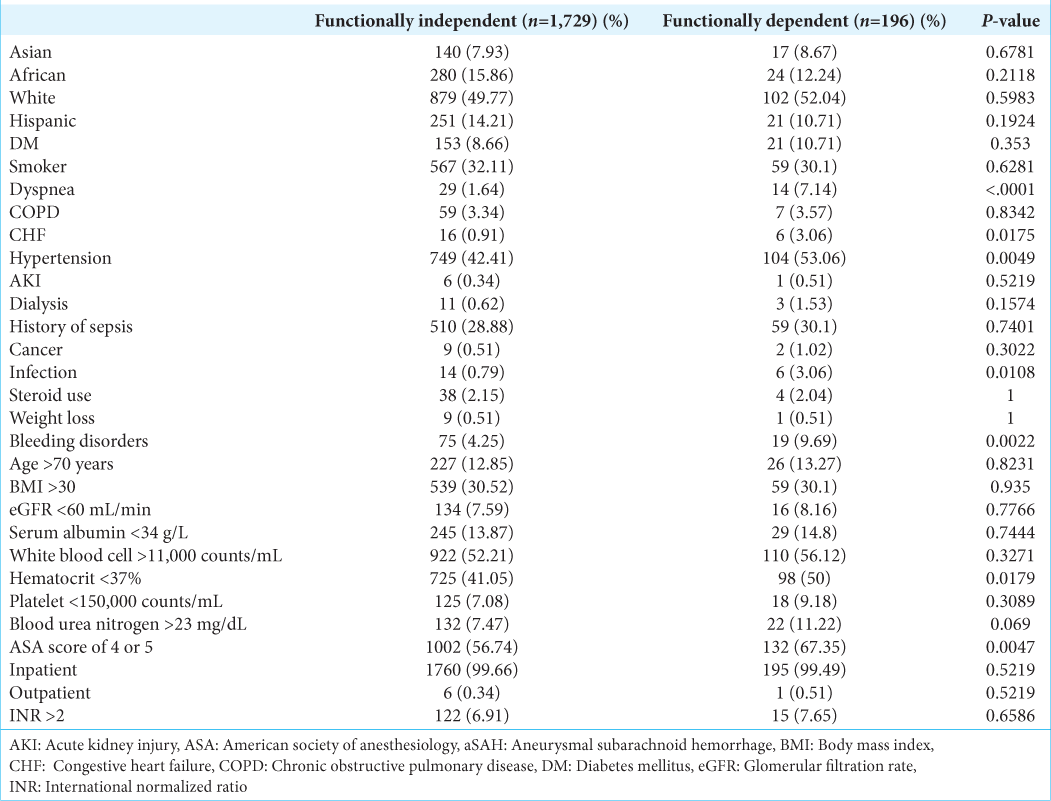
Preoperative variables included several types of patient demographics and comorbidities. Demographics included patient race, age older than 70 years old, smoking history in the past year, body mass index (BMI) >30 kg/m2, and hospital admission status. Comorbidities included dyspnea, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), hypertension, diabetes, acute kidney injury, dialysis, glomerular filtration rate <60 mL/min, preoperative infection, preoperative sepsis, disseminated cancer, usage of steroids, weight loss, bleeding disorders, serum albumin <3.4 g/L, white blood cell count >11,000 counts/mL, hematocrit <37%, platelet count <150,000 counts/mL, blood urea nitrogen >23 mg/dL, American Society of Anesthesiologists (ASA) Classification score of 4 or 5, and international normalized ratio >2. In addition, the aneurysm location was noted by CPT codes (carotid circulation: 61697, 61700; vertebrobasilar circulation: 61698, 61702).
Postoperative variables
Post aSAH-surgery outcomes included mortality, major adverse cardiovascular events (MACE), cardiac events, stroke, wound complications, renal complications, postoperative sepsis, clot formation, pulmonary events, return to the operating room (OR), operation time >4 h, length of stay longer than 7 days, discharge not to home, and postoperative bleeding. MACE refers to a combination of stroke, myocardial infarction, and cardiac arrest, which requires cardiopulmonary resuscitation. Wound complications in this study mean infections at the surgical site and wound dehiscence. Renal complications include progressive renal insufficiency (the National Surgical Quality Improvement Program [NSQIP] defines this as an increase in serum creatinine by >2 mg/dL relative to the preoperative value) and acute renal failure that requires renal replacement therapy. Pneumonia, unplanned reintubation, and mechanical ventilation for longer than 48 hours are what pulmonary events refer to. In addition, this study examined the same surgical outcomes in patients receiving craniotomy for aSAH.
Statistical analysis
Fisher’s exact test was conducted to look for significant preoperative differences, and a multivariable regression was performed between COPD and non-COPD patients to account for the preoperative differences, and those with sufficient differences (P < 0.1) were included in the model. The 30-day postoperative outcomes between the two groups were then compared, and adjusted odds ratios (aOR) were determined. All statistical analyses were conducted using statistical analysis system (SAS) (version 9.4). All analyses were performed using SAS version 9.4. The authors had full access to all data and took responsibility for the integrity of the data analysis. The ACS NSQIP data were accessed from The George Washington University and all subsequent analyses were performed within the institute.
RESULTS
In this study, there were a total of 1925 aSAH patients who underwent neurosurgery for treatment and were stratified into 196 DFS patients and 1729 IFS patients. In the craniotomy subgroup, there were a total of 1546 patients, which were divided into 140 DFS and 1406 IFS patients. Significant preoperative differences were accounted for between the two groups, and the 30-day perioperative outcomes were compared to explore the role of functional status in the post-aSAH neurosurgery outcome.
Preoperative variables for patients receiving any neurosurgical intervention for aSAH are summarized in
Postoperative outcomes for patients receiving any neurosurgical intervention for aSAH are summarized in
DISCUSSION
In our study, we compared 30-day postoperative outcomes for patients receiving craniotomy for an emergent condition (aSAH) between two groups, DFS and IFS patients. We observed higher rates of MACE, cardiac complications, sepsis, pulmonary complications, and discharge not to home among DFS patients compared to IFS patients after craniotomy. We did not see a difference in mortality and bleeding among DFS compared to IFS patients. One’s functional status may be an important preoperative marker to determine outcomes, as this has been documented as an important preoperative marker for various other types of surgeries.[
The average percentage of people over age 60 is expected to increase dramatically over the upcoming decades.[
Previously, functional status has been documented to be a strong indicator of various post-operative complications in procedures related to the vasculature, such as endovascular repair of abdominal aortic aneurysms and carotid stenting.[
In 2017, De La Garza Ramos et al. demonstrated that after adult spinal deformity surgery, DFS patients are at an increased risk of stroke and myocardial infarction compared to IFS patients.[
A possible reason for our observation of no difference in mortality or bleeding is that functional status may impact this variable beyond the 30-day period that we analyzed. In addition, mortality and bleeding in aSAH patients are often due to early re-rupture, but by the time a patient gets a craniotomy, their blood pressure has been controlled in the ICU. Therefore, mortality differences may not be seen as aSAH patients who make it to a craniotomy have controlled blood pressure. In addition, our sample size among craniotomy aSAH patients may not be large enough to detect differences in these variables that may be present. Furthermore, it is possible that due to the selectivity requirements to receive craniotomy, patients receiving this may have unique characteristics different from the general IFS or DFS population that would eliminate any differences in outcomes. Although not precisely the same as functional status, previous literature has demonstrated increased mortality among more frail patients receiving brain tumor resections.[
We additionally observed that DFS patients are less likely than IFS patients to have their aneurysm located in the carotid circulation before craniotomy. DFS patients are more likely to have comorbidities that make a surgeon prefer endovascular approaches versus craniotomy. In addition, surgeons may be more inclined to do an endovascular approach if the aneurysm is located in the posterior circulation, which is not mainly supplied by the carotid arteries.[
Multiple important limitations are present in this retrospective study. First, there is the possibility of unknown or uncontrolled bias. In addition, relevant aSAH variables such as the clinical grade, the Hunt and Hess Scale, the World Federation of Neurological Surgeons scale, the Glasgow outcome scale, the modified Rankin scale, and the size of the aneurysm are not recorded in the NSQIP database. Another variable, endovascular coiling, is not recorded in the NSQIP database. Analyzing postoperative differences in outcomes using endovascular coiling compared to craniotomy could not be accomplished as a result. Furthermore, due to the nature of the NSQIP database exclusively tracking 30-day postoperative outcomes, and not further, outcomes that occur beyond this period following the neurosurgery for aSAH could not be examined.
We adjusted that the data were multiple preoperative variables; however, unrecorded confounding variables may still be present. Additional variables that may have an impact but are not recorded in the NSQIP database are differences in hospital resources, volume, physician experience, and socioeconomic variables (for example, hospital type). In addition, NSQIP does not record if patients were living at home preoperatively, and this may account for differences between DFS and IFS patients in their rates of discharge, not at home. Furthermore, there may be selection bias in NSQIP-participating hospitals. Due to this potential selection bias in NSQIP participating sites, it is possible that this database does not accurately represent the American aSAH population. Postoperatively, other aSAH adverse outcomes may exist that were not recorded in NSQIP and, thus, were not analyzed. Examples include cerebral vasospasm, hydrocephalus, and delayed cerebral ischemia. Furthermore, it is possible that even given the large sample size NSQIP provides, differences in the outcomes analyzed would be detected only if examining larger sample sizes.
Even given the above limitations, this retrospective study establishes a framework for examining the impact of a single variable, functional status, on craniotomy outcomes for aSAH and NSQIP, which has the advantage of having large patient sizes which are unmatched by most institutional datasets. Future investigation is needed using data that can account for the missing preoperative and postoperative variables when comparing post-aSAH surgical outcomes. In addition, future research is needed that examines outcomes beyond the 30-day post-neurosurgery period.
CONCLUSION
In summary, in this study, we observed that dependent functional status significantly increases the risk of MACE, cardiac complications, sepsis, pulmonary complications, and discharge not to home compared to functionally independent patients. Mortality and bleeding risk following craniotomy for aSAH were not significantly different between these groups. We analyzed two different groups of patients with aSAH, DFS, and IFS patients and compared their postoperative outcomes after surgery for aSAH. Our analyses aimed to provide healthcare providers with insights into the risks associated with DFS in patients undergoing surgery for aSAH and plan for corresponding management.
Ethical approval
The Institutional Review Board approval is not required, as it is a retrospective study derived from ACS-NSQIP data.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
The authors acknowledge Dr. Richard Amdur, PhD, for giving statistical support for this project.
References
1. Abulhasan YB, Alabdulraheem N, Simoneau G, Angle MR, Teitelbaum J. Mortality after spontaneous subarachnoid hemorrhage: Causality and validation of a prediction model. World Neurosurg. 2018. 112: e799-811
2. Albright EL, Davenport DL, Roth JS. Preoperative functional health status impacts outcomes after ventral hernia repair. Am Surg. 2012. 78: 230-4
3. de la Fuente SG, Bennett KM, Scarborough JE. Functional status determines postoperative outcomes in elderly patients undergoing hepatic resections. J Surg Oncol. 2013. 107: 865-70
4. De la Garza Ramos R, Goodwin CR, Elder BD, Boah AO, Miller EK, Jain A. Preoperative functional status as a predictor of short-term outcome in adult spinal deformity surgery. J Clin Neurosci. 2017. 39: 118-23
5. de Oliveira Manoel AL. Surgery for spontaneous intracerebral hemorrhage. Crit Care. 2020. 24: 45
6. De Rooij NK, Linn FH, Van Der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007. 78: 1365-72
7. Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011. 27: 27-37
8. Harris DG, Bulatao I, Oates CP, Kalsi R, Drucker CB, Menon N. Functional status predicts major complications and death after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017. 66: 743-50
9. Kanasi E, Ayilavarapu S, Jones J. The aging population: demographics and the biology of aging. Periodontol. 2000 2016. 72: 13-8
10. Lantigua H, Ortega-Gutierrez S, Schmidt JM, Lee K, Badjatia N, Agarwal S. Subarachnoid hemorrhage: Who dies, and why?. Crit Care. 2015. 19: 309
11. Lee KS, Zhang JJ, Nguyen V, Han J, Johnson JN, Kirollos R. The evolution of intracranial aneurysm treatment techniques and future directions. Neurosurg Rev. 2022. 45: 1-25
12. Leven DM, Lee NJ, Kim JS, Kothari P, Steinberger J, Guzman J. Frailty is predictive of adverse postoperative events in patients undergoing lumbar fusion. Glob Spine J. 2017. 7: 529-35
13. Loftus CM. Diagnosis and management of nontraumatic subarachnoid hemorrhage in elderly patients. Clin Geriatr Med. 1991. 7: 569-82
14. Marder CP, Narla V, Fink JR, Tozer Fink KR. Subarachnoid hemorrhage: Beyond aneurysms. AJR Am J Roentgenol. 2014. 202: 25-37
15. Mounsey M, Gillis A, Ata A, Vignaly L, Stain SC, Tafen M. Dependent status is a risk factor for complications after thyroidectomy. Am J Surg. 2022. 224: 1034-7
16. Muehlschlegel S. Subarachnoid hemorrhage. Continuum (Minneap Minn). 2018. 24: 1623-57
17. Sastry RA, Pertsch NJ, Tang O, Shao B, Toms SA, Weil RJ. Frailty and outcomes after craniotomy for brain tumor. J Clin Neurosci. 2020. 81: 95-100
18. Scarborough JE, Bennett KM, Englum BR, Pappas TN, LagooDeenadayalan SA. The impact of functional dependency on outcomes after complex general and vascular surgery. Ann Surg. 2015. 261: 432-7
19. Skube SJ, Lindemann EA, Arsoniadis EG, Akre M, Wick EC, Melton GB. Characterizing functional health status of surgical patients in clinical notes. AMIA Jt Summits Transl Sci Proc. 2018. 2018: 379-88
20. Thommen R, Kazim SF, Rumalla K, Kassicieh AJ, Kalakoti P, Schmidt MH. Preoperative frailty measured by risk analysis index predicts complications and poor discharge outcomes after Brain Tumor Resection in a large multi-center analysis. J Neurooncol. 2022. 160: 285-97
21. Van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain. 2001. 124: 249-78
22. Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995. 273: 59-65
23. Zil-E-Ali A, Kusztos V, Flohr TR, Aziz F. Preoperative dependent functional status is associated with poor outcomes after carotid endarterectomy and carotid stenting in both symptomatic and asymptomatic patients. Ann Vasc Surg. 2021. 76: 114-27